Vapour Pressure of Solution
Francois Marie Raoult discovered the vapour of solution in the end of the 19th century. And his work was published in the years of 1887 to 1888 by which everyone got to know about Raoult's law. François-Marie Raoult, was a french chemist and studied about the vapour pressure. He observed that when a non-volatile solute is added to the solvent the vapour of the solution changes. This is what he states in his discovery which is Raoult's law in this law he states that the when vapour pressure of solvent in the ideal solution is directly proportional to the mole fraction of the solvent in the solution.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Vapour Pressure
- Factors On Which Vapour Pressure Depends
- Some Solved Examples
- Summary
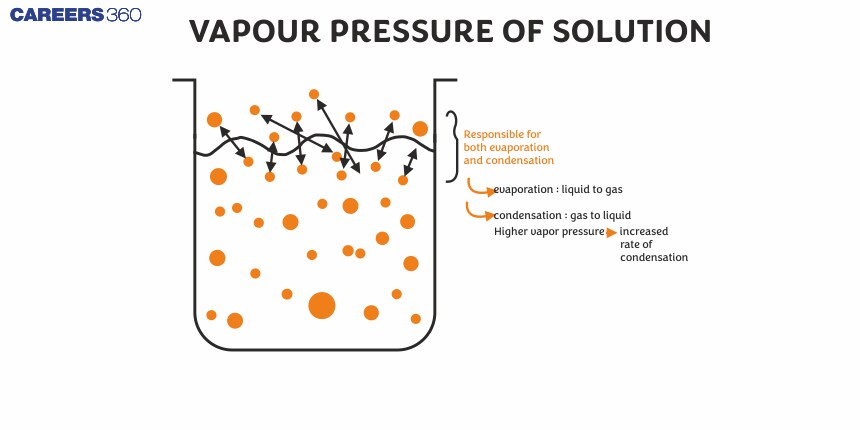
He experimented on water and benzene and measured their vapour pressure by adding different non-volatile solutions to them. In performing this series of experiments he demonstrated that the addition of a non-volatile solute decreased the vapour pressure of the solvent, which he aligned with his law. Raoult's law is a Solutions chemistry framework and has various applications in the scientific and industrial fields. He provides the quantitative method For knowing the behaviour of the solution, which is very sensitive in drug formulations, manufacturing of chemicals and in the field of material science.
Vapour Pressure
It is the pressure exerted by vapours of a pure liquid over its surface when they are in equilibrium with the liquid at a given temperature.
For example, if we take the case of water, then the equilibrium constant of the following physical process will represent the Vapor Pressure of Water (Also sometimes called as Aqueous Tension)
H2O(l))⇌H2O(g),Kp=Po
At equilibrium, the rate of vaporisation = the rate of condensation and the equilibrium constant of the above vapour-liquid equilibrium represents the vapour pressure of the liquid.
It depends upon the nature of the liquid and temperature. Pure liquid has always a vapour pressure greater than its solution.
The vapour pressure of a liquid helps us to have an idea of forces of attraction amongst the molecules of liquid that is, the more the force of attraction, the lower the vapour pressure and vice versa.
The vapour pressure of a liquid increases with an increase in temperature due to an increase in the kinetic energy of solvent molecules that is, an increase in evaporation however it is independent of the nature of the vessel.
Vapour Pressure of a Solution
When a miscible solute is added to a pure solvent, it results in the formation of a solution. As some molecules of solute will replace the molecules of the solvent from the surface, therefore, escaping tendency of solvent molecules decreases. This causes a lowering of vapour pressure.
- The vapour pressure of a solution is less than that of the pure solvent.
- If the vapour pressure of the solvent is P and that of the solution is Ps then lowering of V.P = P - Ps.
- The vapour pressure of the solution decreases as the surface area occupied by the solvent molecule decreases and density increases.
Factors On Which Vapour Pressure Depends
Factors governing the vapour pressure:
1. Temperature: As Temperature increases, the Kinetic Energy of the molecules in the liquid phase increases and as a result, more molecules are able to escape to the gaseous phase and hence the vapour Pressure increases.
The Clausius Clapeyron Equation relates the vapour pressure of the liquid to the temperature and is given as
ln(P2P1)=ΔHR(1 T1−1 T2)
Where ΔH is the heat of vaporisation of the liquid and P1 and P2 are the vapour pressure at temperature T1 and T2 respectively.
2. Vapour pressure depends on the nature of the liquid. The greater the force of attraction between the liquid molecules, the lesser is the vapour pressure
3. vapour pressure does not depend on the shape or the size of the container and has a fixed value at a particular temperature.
Significance of vapour pressure:
- Vapour pressure gives us an idea of intermolecular forces of attraction in the liquid. The greater the force of attraction, the lower is the vapour pressure and vice versa.
Vapour pressure gives us an idea of the volatility (vapour-forming tendency of the liquid). The greater the vapour pressure, the greater is the volatility of the liquid.
Vapour pressure also gives an idea of the boiling point of the liquid. The greater the vapour pressure, the lesser is the boiling point of the liquid.
Recommended topic video on (Vapour Pressure of Solution)
Some Solved Examples
Example.1
1. When a substance is dissolved in a solvent the vapour pressure of the solvent is decreased. This results in:
1) (correct)An increase in the b.p. of the solution
2)A decrease in the b.p. of the solvent
3)The solution has a higher freezing point than the solvent
4)The solution has a lower osmotic pressure than the solvent
Solution
Vapour pressure α1 Boiling point
When vapour pressure decreases then b.pt. increases.
Hence, the answer is the option (1).
Example.2
2. An aqueous solution of methanol in water has vapour pressure:
1)Equal to that of water
2)Less than that of water
3) (correct)More than that of water
4)We can't say
Solution
Methanol has a lower boiling point than H2O.
The lower is the boiling point of the solvent more is vapour pressure.
Hence, the answer is the option (3).
Example.3
3. Which has the maximum vapour pressure?
1)HI
2)HBr
3) (correct)HCl
4)HF
Solution
The lower the boiling point the greater is vapour pressure.
boiling point order: HCl < HBr < HI < HF
Hence, the answer is the option (3).
Example.4
4. A mixture of 100 m mol of Ca(OH)2 and 2 g of sodium sulphate was dissolved in water and the volume was made up to 100 mL.The mass of calcium sulphate formed and the concentration of in the resulting solution, respectively , are : (Molar mass of Ca(OH)2,Na2SO4 and CaSO4 , are 74, 143 and 136 g mol−1 , respectively ; Ksp of Ca(OH)2 is 5.5×10−6,)
1) (correct)1.9 g,0.28 mol L−1
2)13.6 g,0.28molL−1
3)1.9 g,0.14molL−1
4)13.6 g⋅0.14 mol−1
Solution
Given,
Mol of Na2SO4 = 2/142 = 14 m mol
Ca(OH)2+Na2SO4⟶CaSO4+2NaOHmmol1001414 m/mol28 m/mol
Mass of CaSO4=14×1361000=1.9gm
Molarity of OH−=28100=0.28 mol/L
Example.5
5. Which of the following has the highest vapour Pressure at Room temperature?
1)Benzene
2)Toluene
3)n-Hexane
4) (correct)Naphthalene
Solution
Vapour Pressure α Surface area
α Molecular Mass
Among the given compounds naphthalene has a higher molecular Mass.
Hence, the answer is the option (4).
Example.6
6. Three solutions are prepared by adding ‘w’ g of ‘A’ into 1 kg of water, ‘w’ g of ‘B’ into another 1 kg of water and ‘w’ g of ‘C’ in another 1 kg of water (A, B, C are non-electrolytic). Dry air is passed from these solutions in sequence (A B
C). The loss in weight of solution A was found to be 2 g while solution B gained 0.5 g and solution C lost 1 g. Then the relation between molar masses of A,B and C is :
1)MA:MB:MC=4:3:5
2)MA:MB:MC=14:13:15
3) (correct)MC>MA>MB
4),MB>MA>MC
Solution
The loss in weight from A is due to dry air and proportional to vapour pressure above that solution. The loss or gain in mass from solution B or C will depend upon the Pressure above that solution and the pressure inside the solution from which it is coming into the particular solution.
So,
PA∝2gm
(PA−PB)∝0.5gm(PC−PB)∝2.5gm
Hence, the order of Pressure: PC > PA> PB
So, the maximum vapour pressure is above solution C hence, it has minimum lowering and hence the minimum mole fraction (hence a minimum number of moles) of solute So, it has a maximum molar mass of the substance.
Hence, the answer is the option (3).
Summary
The vapour pressure of a solution is a fundamental concept in chemistry which tells the capability of liquid to change in the gaseous phase means that vapour pressure is the pressure in which the liquid can change into the gaseous state. This property is essential for understanding various phenomena and processes in both theoretical and practical fields. Vapour pressure has a very important role in predicting the boiling point of any solution, which is the temperature at which its vapour pressure equals the atmospheric pressure. As a result, solutions with lower vapour pressures generally have higher boiling points compared to their pure solvents.
Also Read
19 Feb'25 06:42 PM
19 Feb'25 06:38 PM
19 Feb'25 06:35 PM
19 Feb'25 06:29 PM
19 Feb'25 06:23 PM
19 Feb'25 05:11 PM
25 Oct'24 05:23 PM
18 Oct'24 10:46 AM
10 Oct'24 12:09 PM
10 Oct'24 12:06 PM