Alpha decay : Definition, Example, & Facts
A nuclear process known as "alpha decay" releases a particle made up of two neutrons and two protons when an unstable nucleus transforms into another element. This ejected substance is referred described as an "alpha particle.". Ernest Rutherford investigated how radiation is deflected through magnetism to distinguish alpha decay from other types of radiation. Given that the alpha particles carry a +2e charge, the alpha decay would deflect a positive charge.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Describe Alpha Decay
- Uses Of Alpha Decay
- The Equation For Alpha Decay
- How Does Alpha Decay Work?
- Gamow's Alpha Decay Theory
- Protection
- History
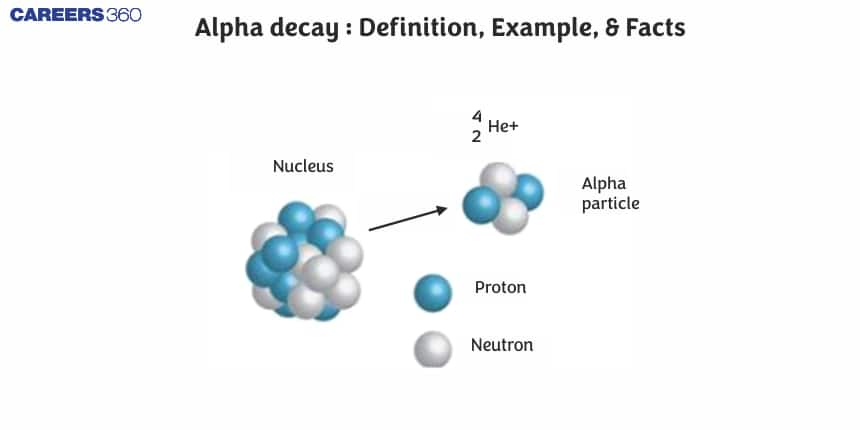
Describe Alpha Decay
A nuclear process known as "alpha decay" releases a particle made up of two neutrons and two protons when an unstable nucleus transforms into another element. The helium nucleus that is being ejected is termed an “alpha particle”. Positive charge and a sizable mass characterise alpha particles. Due to their high mass, alpha particles are unable to penetrate solids or the atmosphere very deeply. Alpha decay is rarely employed in requires medical radiation therapy because alpha particles only have an impact on surfaces.
Ernest Rutherford first identified alpha decay as distinct from other radiation types by studying the radiation's refraction via a magnetic field. Since alpha particles carry a+2e charge, alpha decay deflects as you would anticipate a positive particle
Uses Of Alpha Decay
Uses of alpha decay are:
Smoke detectors employ the alpha emitter americium-241. In an open ion chamber, the alpha particles ionise the air, which then experiences a little amount of current. The smoke detector sounds an alarm when smoke embers from the fire reach the chamber, decreasing the current.
Additionally, an alpha emitter is radium-223. It is employed to treat bone tumours (cancers in the bones).
Radioactive substances thermoelectric generators used in space missions and artificial heart pacemakers can be safely powered by alpha decay. Other types of radioactive decay are far more difficult to protect against than alpha decay.
Polonium-210, an alpha absorber, is frequently used in static eliminators to ionise the air, causing the "static cling" to disperse more quickly.
The Equation For Alpha Decay
While the atomic number decreases by two during -decay, the nuclear reaction's output nucleus' mass number is four less than the atomic nucleus. The alpha decay equation is typically shown as follows:
{}_{Z}^{A}X\to {}_{Z-2}^{A-4}\,Y\,+\,{}_{2}^{4}\,He
![]()
where:
The parent nucleus, or the initial nucleus, is {}_{Z}^{A}X
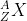 .
.The number of nucleons overall is A.
The number of protons overall is Z.
The offspring nucleus, or terminating nucleus, is { }_{Z-2}^{A-4} Y
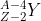 .
.The expelled alpha particle is { }_2^4 \mathrm{He}
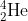 .
.
How Does Alpha Decay Work?
A typical radioactive decay process when a nucleus releases an alpha particle (a helium-4 nucleus). The nucleus releases an alpha particle or helium nucleus during alpha decay. Massive nuclei with a high proton-to-neutron ratio experience alpha decay. The parent nucleus becomes more stable as a result of alpha radiation's reduction of the protons to neutrons ratio.
Gamow's Alpha Decay Theory
The decay frequency of a radioactive element and the strength of the released alpha particles are related by the Geiger-Nuttall law or rule. In accordance with this relationship, half-lives and decay energy are exponentially related, meaning that very significant changes in half-life correspond to very modest changes in decay energy and, therefore, alpha particle energy.
This law dictates that short-lived isotopes release higher powerful alpha particles than long-lived isotopes. The name of this law was given in honour of Hans Geiger and John Mitchell Nuttall, two physicists who first proposed it in 1911.
Protection
The consumption of a material that experiences alpha decay is dangerous even when it doesn't penetrate very deeply since the released alpha particles can quickly cause internal tissue damage despite their limited range. Interaction with membranes and live cells led to this harm.
Depending on how the exposure occurs, alpha particles have different health implications. There may be long-lasting biological harm if the alpha emitter is ingested, absorbed, or absorbed into the body's bloodstream. The risk of cancer is raised by this damage. If the alpha emitter is breathed, alpha radiation is well-known to cause lung cancer in people. One of the main causes of alpha decay illnesses in humans is the intake of radon, an alpha emitter.
History
Ernest Rutherford originally mentioned alpha particles in his studies on radioactivity in 1899; by 1907, they had been recognized as He2+ ions. George Gamow had discovered a tunnelling solution to the idea of alpha decay by 1928. An inviting nuclear potential well and a repellent electromagnetic potential barrier hold the alpha particle inside the nucleus. The capacity to leave the nucleus is traditionally prohibited, but according to the rules of quantum mechanics, there is a very small chance of doing so.
Gamow derived the Geiger-Nuttall law, which had previously been discovered empirically, by solving a virtual potential for the nucleus and establishing from first principles the link between both the half-life of the emission and the energy of the decay.
Frequently Asked Questions (FAQs)
A positive charge particle similar to the helium-4 nucleus is spontaneously released during alpha decay. Two protons and two neutrons make up this particle, also referred to as an alpha particle. Sir Ernest Rutherford made the discovery and gave it a name in 1899.
The distinctions between radioactive decay of the alpha, beta, and gamma rays can be summed up as follows: Beta decay produces a new element that has one more proton and one fewer neutron while alpha decay creates a new element with some fewer protons and two fewer neutrons.
In general, there are two different kinds of natural radioactive decay: alpha decay, which is produced when radon gas emits particles "containing two neutrons and two protons," and nuclear decay caused by photon emission.
The positive charge particles are called alpha rays. The helium atom that makes up an alpha particle is extremely active and energetic and has two neutrons and protons. The least penetrating and most ionising particles are these. They have a high ionisation power, which means that if they enter the body, they can cause severe harm.
Strong nuclear force and electromagnetic force are the two forces that work together to produce alpha decay. The strong nuclear force is a force of attraction that not only holds quarks together in the nucleus to create protons and neutrons as well as other forms of particles.
Also Read
28 Nov'24 01:14 AM
16 Nov'24 01:37 PM
14 Nov'24 05:50 PM
12 Nov'24 01:15 AM
24 Sep'24 04:35 PM