Deuteron Mass - Definition, Properties, MeV, AMU, FAQs
Did you know that deuterons are found in a type of hydrogen which is called heavy hydrogen, and have some amazing real-life uses? They help us create clean energy by powering fusion reactors and are also used in cancer treatment in which they help to destroy harmful cell without affecting the healthy ones and many more. In this article, we are going to study about deuterons definition history and more. Keep continuing with the article to know in depth about deuterons
This Story also Contains
- What is Deuteron
- Mass of Deuterium
- Difference Between Deuteron and Deuterium
- History of Deutron
- Properties of Deuteron
- Deuteron Mass and Charge
- Measurement of Binding Energy of Deuteron
- Applications of Deuteron Mass

What is Deuteron
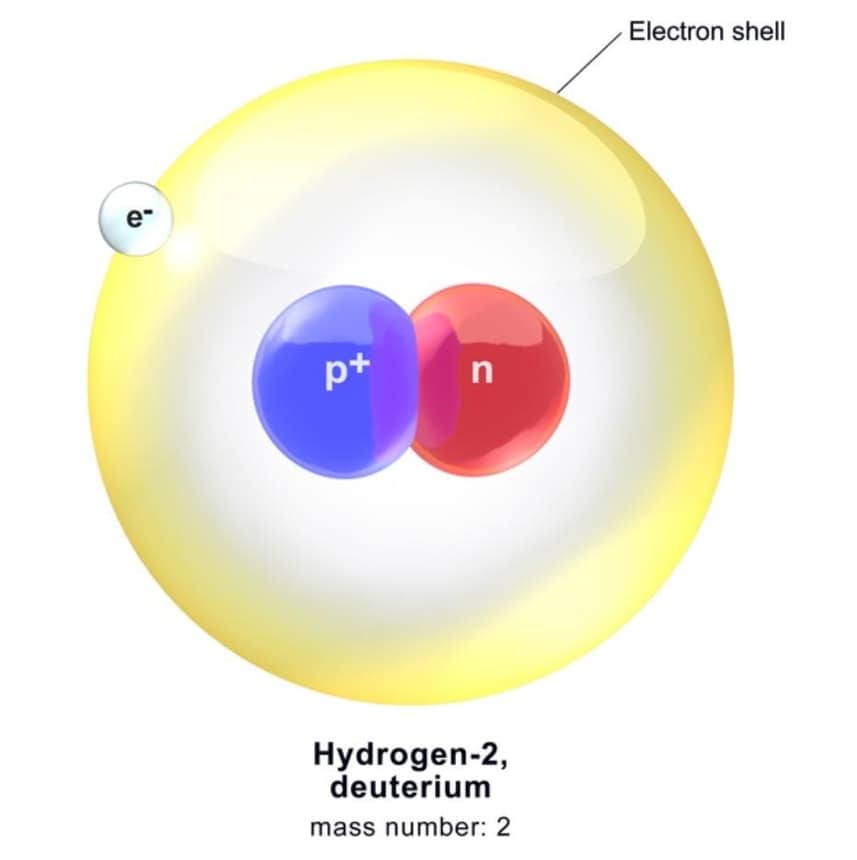
A deuteron is a very small (tiny) part of an atom, which is a type of hydrogen atom called heavy hydrogen or deuterium. Deutron has one neutron and one proton inside it.
Deuteron symbol is represented as 12D. A non-aligned atom of 2H is termed as deuterium. It is the unpretentious bound state of nucleons and thus gives us an ideal system for learning the nucleon-nucleon interaction. For nuclear physicists, the deuterons should be what the hydrogen atoms are for atomic physicists.
Mass of Deuterium
$
\begin{array}{|l|l|}
\hline \text { Form of Mass } & \text { Value } \\
\hline \text { Mass in Atomic Mass Units (amu) } & 2.013553212745(40) \mathrm{u} \\
\hline \text { Mass in MeV/c² } & 1875.612928(12) \mathrm{MeV} / \mathrm{c}^2 \\
\hline \text { Mass in Kilograms (kg) } & 3.3435837724 \times 10^{-27} \mathrm{~kg} \\
\hline \text { Mass in Grams (g) } & 3.3435837724 \times 10^{-24} \mathrm{~g} \\
\hline \text { Mass in Joules (J) (via } E=m c^2 \text { ) } & 3.005 \times 10^{-10} \mathrm{~J} \\
\hline \text { Mass in Electron Volts (eV) } & 1.875612928 \times 10^9 \mathrm{eV} \\
\hline \text { Mass in Energy Equivalent (GeV) } & 1.875612928 \mathrm{GeV} \\
\hline
\end{array}
$
Difference Between Deuteron and Deuterium
| Deuteron | Deuterium |
| The nucleus of deuterium, consisting of one proton and one neutron. |
An isotope of hydrogen with one proton, one neutron, and one electron. |
| ²H⁺ or d (for deuteron nucleus, positively charged). |
²H or D (for deuterium atom, neutral charge). |
| Positively charged (as it lacks the electron). |
Electrically neutral (proton and electron balance). |
| 1 proton and 1 neutron (no electron). |
1 proton, 1 neutron, and 1 electron. |
History of Deutron
Deuteron gets its name from the Greek word “deuteros”, which means “second”. It refers to the two particles that make up the nucleus. Harold Urey first discovered it in 1931. Deuteron’s stability has been noteworthy since its discovery.
Properties of Deuteron
- Composition: A deuteron is the nucleus of deuterium (heavy hydrogen) and is made up of one proton and one neutron.
- Charge: Since it has one proton, its charge is $\mathbf{+ 1 e}$, the same as that of a proton.
- Mass: The mass of a deuteron is approximately $\mathbf{3 . 3 4 3} \times \mathbf{1 0}^{\mathbf{- 2 7}} \mathbf{~ k g}$, which is slightly less than the combined mass of a free proton and neutron due to binding energy.
- Spin: A deuteron has a spin quantum number of 1, meaning it is a boson.
- Magnetic Moment: The magnetic dipole moment of a deuteron is 0.857 nuclear magnetons ( $\mu_n$ ).
- Binding Energy: The binding energy of the deuteron is $\mathbf{2 . 2 2 4} \mathbf{~ M e V}$, showing how strongly the proton and neutron are held together.
- Stability: Deuterons are stable and do not undergo radioactive decay.
Deuteron Mass and Charge
After knowing about properties of deuteron, now move to the mass and charge of deuteron.
Let us explain deuteron Mass and Charge. Deuterium is an atomic particle with a proton and neutron that is stable. Hydrogen-2 is indicated by the letters D or 2H. The mass of a deuteron is measured in atomic mass units (2.0135 amu) or million electron volts (1875.612928 MeV). The deuteron has a charge of +1e. This is because protons are present.
Measurement of Binding Energy of Deuteron
The binding energy of a deuteron is the amount of energy required to separate it into its two particles one proton and one neutron. It tells us how strongly these particles are held together in the nucleus.
To measure this energy, we use the mass defect method.
1. Find the masses:
- Mass of proton $=1.007825 \mathrm{u}$
- Mass of neutron $=1.008665 \mathrm{u}$
- Mass of deuteron $=2.014102 \mathrm{u}$
2. Calculate mass defect ( $\Delta \mathrm{m}$ ):
$
\begin{gathered}
\Delta m=\left(m_p+m_n\right)-m_d \\
\Delta m=(1.007825+1.008665)-2.014102=0.002388 u
\end{gathered}
$
3. Convert mass defect to energy:
$
1 u=931.5 \mathrm{MeV}
$
$
\text { Binding Energy }(\mathrm{B})=0.002388 \times 931.5=2.224 \mathrm{MeV}
$
Applications of Deuteron Mass
Nuclear Fusion Research:
- Key role in fusion reactions like deuterium-tritium fusion.
- Studied in reactors such as ITER for clean energy production.
Medical Applications:
- Used in neutron therapy for cancer treatment.
- Deuteron mass helps calculate the energy levels required.
Particle Accelerators:
- Deuterons are accelerated for nuclear physics experiments.
- Mass is crucial for determining energy and speed in accelerators.
Nuclear Reaction Calculations:
- Vital for calculating binding energy and reaction rates.
- Helps understand energy output in fusion and fission processes.
Also check-
Frequently Asked Questions (FAQs)
A deuteron (2H nucleus) comprises of a neutron and a proton. (A non-aligned atom of 2H is termed as deuterium.). It is the unpretentious bound state of nucleons and thus gives us an ideal system for learning the nucleon-nucleon interaction. Deuteron symbol is represented as 12D.
Mass of deuteron in kg is 3.343 x 10-27 kg.
Deuteron’s binding energy is given by 2.22463 MeV
Harold Urey first discovered it in 1931.
The quadruple moment of deuteron is determined by radio frequency molecular beam method.
