Difference Between Conduction Convection and Radiation
Heat transfer is an important concept in physics and daily life. Whenever two objects or regions have different temperatures, heat flows from the hotter object to the colder one. This transfer of heat happens through three primary methods: conduction, convection, and radiation. In this article, we will explain each method of heat transfer with easy examples and also discuss the difference between conduction, convection and radiation in a simple comparison table.
This Story also Contains
- What is Heat Transfer?
- Difference Between Conduction Convection and Radiation:
- Methods Of Heat Transfer
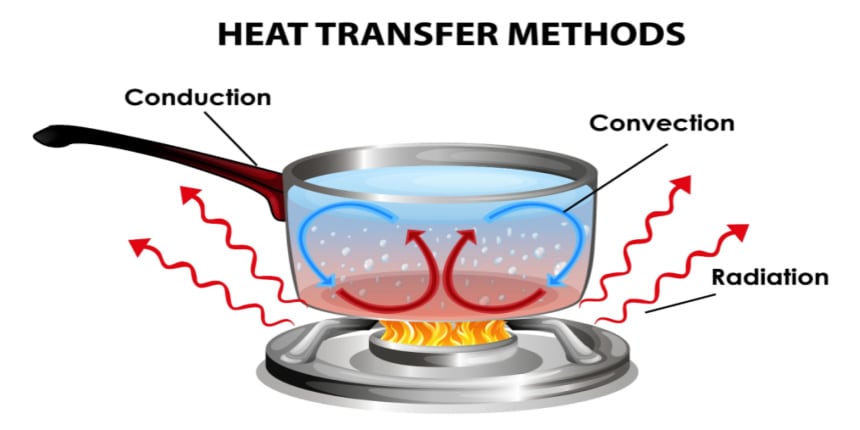
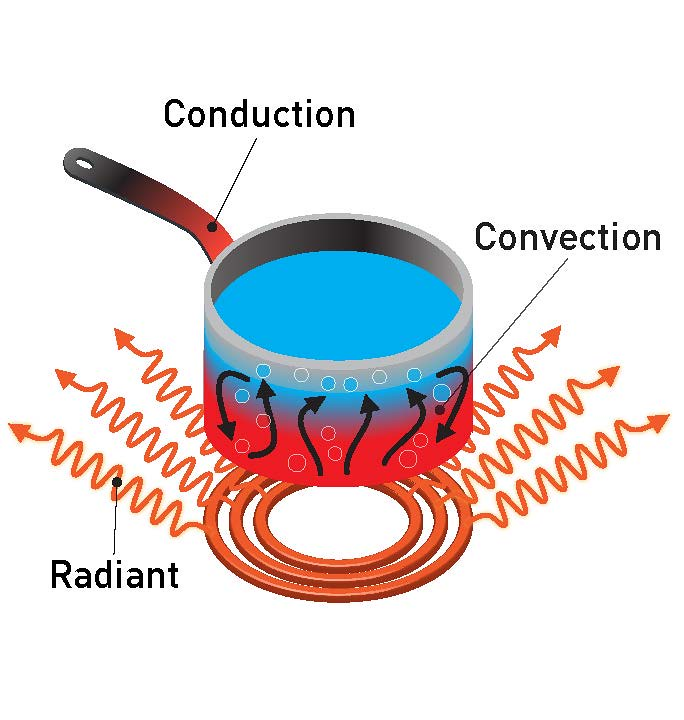
- Conduction
- Convection
- Radiation
Also, check-
What is Heat Transfer?
Heat transfer is the movement of thermal energy from one place to another due to a temperature difference. Depending on the medium (solid, liquid, gas, or vacuum), heat is transferred through:
- Conduction - direct contact
- Convection - movement of fluid
- Radiation - electromagnetic waves
Difference Between Conduction Convection and Radiation:
| Conduction | Convection | Radiation |
| In the process of conduction, heat gets transferred between substances with direct contact with solids | In the process of conduction, heat gets transferred between substances like fluid | In the process of radiation, heat gets transferred through the means of electromagnetic waves without any interaction between particles. |
| The process is carried out by the electrons | The process occurs mainly due to the movement of the molecules | The process is carried out in the form of the Em waves |
| The transfer of heat occurs due to temperature difference | The transfer of heat occurs due to the density difference between the fluids | The transfer of heat takes place in objects which have temperatures higher than 0K |
| In conduction, the transfer of heat is a slow process | In convection, the transfer of heat is faster than in conduction | In radiation, the transfer of heat is the fastest process compared with conduction and convection |
| The transfer of heat through some solid heated object | The transfer of heat through some intermediate objects. | The transfer of heat happens through the Em waves |
| The conduction process will not obey the laws of reflection and the laws of refraction. | The convection process will not obey the laws of reflection and the laws of refraction. | The conduction process will obey the laws of reflection and the laws of refraction. |
| The conduction process continues until both substances are maintained under an equal temperature | The convection process occurs only when the temperature difference occurs between the objects. | Radiation processes at any condition as the energy can be transferred through space. |
| This process occurs only in solid matter. | This process occurs in the fluid. (includes both solids and liquids)) | This process takes place in all matters like solids, liquids, and gases which have a temperature above 0k. |
Interested students can also check the following links. here they can find NCERT solutions for all class.
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
Methods Of Heat Transfer
Do you know how heat transfers from one object to another object? There are mainly three different modes of heat transfer which are listed below.
- Conduction
- Convection
- Radiation
Conduction
Conduction is the transfer of heat through direct contact between particles. It mainly occurs in solids, especially metals, because they have free electrons that transfer heat quickly.
Everyday Examples of Conduction
- A metal spoon getting hot when placed in hot soup
- Heat passing through a cooking pan
- Ironing clothes
- Walking barefoot on hot sand
Important Points
- No movement of material
- Depends on thermal conductivity of a substance
- Heat flows from higher temperature to lower temperature
Convection
Convection occurs in fluids (liquids and gases). Heat is transferred by the motion of particles, which form convection currents.
Everyday Examples of Convection
- Boiling water
- Hot air balloon rising
- Sea breeze and land breeze
- Room heater warming the air
Types of Convection
- Natural Convection - caused by density differences due to heating
- Forced Convection - uses external agents like fans, pumps or blowers
Also read :
Radiation
Heat transfer is which the heat can be transferred in the form of electromagnetic waves. This process does not require any medium for energy transfer. There is no requirement for any direct contact or molecule movement for this process. It occurs without touching the object. Radiation depends on some kind of surface properties like color, the orientation of the surface, and so on. The energy carried out by the EM waves is known as radiant energy. This energy can travel through a vacuum from the source to its surroundings. Example: The heat from the sun that reaches earth takes the form of EM waves and this is one of the radiation waves.
Also, there are some important related topics. interested students can read to know more about the topics.
- Calorimeter
- Unit of Heat
- Relation Between Calorie and Joule
- Difference Between Heat and Temperature
- Relation Between Celsius and Fahrenheit
NCERT Physics Notes :
Frequently Asked Questions (FAQs)
Thermal radiations are referred to as radiant heat. Thermal radiation is generated by the emission of electromagnetic waves. These waves carry away the energy from the emitting body. Radiation takes place through a vacuum or transparent medium which can be either solid or liquid
These properties include thermal conductivity, density, viscosity, melting point/glass transition temperature, and heat capacity
Convection is a mode of heat transfer in fluids. in water heat flow through the convection.
following are three types of heat transfer.
- radiation.
- conduction.
- convection.
Conduction | Convection | Radiation |
In the process of conduction, heat gets transferred between substances with direct contact with solids | In the process of conduction, heat gets transferred between substances like fluid | In the process of radiation, heat gets transferred through the means of electromagnetic waves without any interaction between particles. |
This process occurs only in solid matter. | This process occurs in the fluid. (includes both solids and liquids)) | This process takes place in all matters like solid, liquid, gases which have a temperature above 0k. |
Conduction:
The heat transfer in any solid body (without any radiation flow) occurs due to collision between the higher energy molecules with lower energy molecules is known as conduction. The collision occurs due to the free electrons in the solids. Example: Metals are good conductors and heat conduction is easily found in metals.
Convection:
The heat transfer that occurs in fluid due to the movement of the molecules in the fluid is known as convection. The hot object moves upward and the cold object sinks. Example: Air heating systems.
The Convection current happens when the charged particles move as a response to forces that are given mechanically and it is not caused in response to the electric field. The Conduction current occurs when the charged particles move as a response to the electric field and it is not caused by any kind of mechanical forces or other forces.
