Heat Transfer Conduction Convection and Radiation - Types, Example, FAQs
Heat transfer is the process defined in physics that deals with the transfer of thermal energy from one body or region to another. This occurs in three main methods; conduction, convection and radiation. Conduction is basically the transmission of heat within an object or between objects that are in direct contact with each other; for example, a metal spoon gets warm when left in a pot containing boiling water. On the other hand, radiative heat transfer is the transfer of heat in the form of electromagnetic radiation such as heat from the sun or a burning bonfire. All of these mechanisms are not just theoretical notions, but they are instead used on a day-to-day basis when making food constructing the best architectural models or in the analysis of the weather and climatic system.
This Story also Contains
- What is Heat Transfer?
- Types of Heat Transfer
- What is Conduction Heat Transfer?
- What is Convection Heat Transfer?
- What is Radiation Heat Transfer?
- Factors Affecting Heat Transfer
What is Heat Transfer?
Heat can be exchanged between atoms and molecules in any material. The atoms are in a variety of states of motion at any given time. Heat, also known as thermal energy, is created by the motion of molecules and atoms and is found in all matter. The heat energy is proportional to the amount of molecular mobility. Heat transfer takes place, on the other hand, simply by transferring heat from a high-temperature body to a low-temperature one.
According to thermodynamics Heat transfer is defined as the flow of heat across a system's border is due to a temperature difference between the system and its surroundings.
Surprisingly, the temperature differential is said to represent a "potential" that causes heat to travel from one location to another.
Also, read
Types of Heat Transfer
In our daily lives, we've noticed that when a pan is filled with water and placed over a flame, the temperature rises. When the flame is turned off, however, it gradually cools.
This is due to the phenomenon of heat transmission between the water-filled pan and the flame. It has been established that heat is transferred from hotter to colder objects.
When things descend at different temperatures or if an object is at a different temperature than the surroundings, heat is transferred so that both the object and the surroundings achieve a temperature of equilibrium.
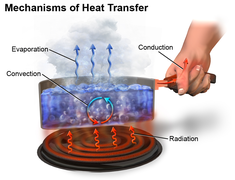
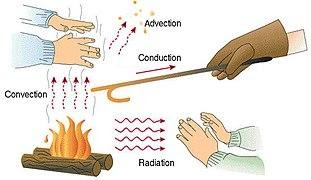
There are three different types of heat transmission. The following are some examples of heat transmission modes- conduction convection radiation.
What is Conduction Heat Transfer?
Heat is transported from a hotter region of the body to a colder area of the body through conduction heat transfer, which involves no actual movement of body molecules. Heat is transferred from one molecule to another as a result of the molecules' vibratory motion. Heat is transferred by the process of conduction heat transfer, which occurs when two substances are in direct touch. In most cases, it occurs in solids.
When frying vegetables in a pan, heat is transferred from the flame to the pan and then next to the veggies.
Substances are classified as conductors or insulators based on their heat conductivity. Conductors are substances that conduct heat swiftly, while insulators are substances that do not conduct heat.
Example of Conduction Heat Transfer
The following are some conduction example
- Clothing ironing is an example of conduction heat transfer, in which heat is transferred from the iron to the clothing.
- When you hold an ice cube in your hands, heat is transferred from your hands to the ice cube, causing it to melt.
- At the beach, heat is transferred through the sand. This is something that can be experienced during the summer. Sand is a good heat conductor.
What is Convection Heat Transfer?
This is a process in which heat is transferred from a higher temperature zone to a lower temperature region in both liquids and gases. Convection heat transfer occurs in part as a result of molecular movement and in part as a result of mass transfer.
Example of Convection Heat Transfer
The following are some examples of convection heat transfer:
- When water boils, the molecules that are denser sink to the bottom and the molecules that are less dense rise, resulting in a circular motion of the molecules, which heats the water.
- Warm water goes towards the poles as it approaches the equator, while cooler water moves towards the equator.
- Warm-blooded animals use convection to circulate their blood, which helps to regulate their body temperature.
What is Radiation Heat Transfer?
Radiation heat transfer is the mechanism through which heat is transported from one body to another without the use of medium molecules. The medium has no bearing on this form of heat transmission.
In an oven, the substances are heated directly without the use of a heating medium, which is one of the ways of heat transfer.
Example of Radiation Heat Transfer
The following are some radiation heat transfer examples:
- In the oven, microwave radiation is an example of radiation.
- The sun's ultraviolet (UV) rays are an example of radiation.
- Radiation is produced as Uranium-238 decays into Thorium-234, as alpha particles are released.
Factors Affecting Heat Transfer
Temperature Difference: The rate of heat transfer increases with an increase in the temperature difference between two bodies.
Nature of Material: Heat is transferred faster through good conductors than through poor conductors.
Area of Contact: Greater the surface area in contact, greater is the amount of heat transferred.
Thickness of Material: Increase in thickness of a material reduces the rate of heat transfer.
Medium of Transfer: Heat transfer depends on the nature of the medium such as solid, liquid, gas, or vacuum.
Time of Contact: The longer the bodies remain in contact, the more heat is transferred.
Frequently Asked Questions (FAQs)
The following are the many heat transfer modes:
Conduction heat transfer
Convection
Radiation heat transfer
UV rays, for example, are emitted by the sun.
In the oven, microwave radiation is emitted.
Joules is the SI unit of heat.
The movement of charged electrons and protons produces electromagnetic radiation.
Convection is the term for this phenomenon.
Convection is a considerably more efficient way of heat transmission in fluids like water and air than conduction. The difference in efficiency resulted in a significant variation in the amount of time it took to melt the ice. Despite the fact that conduction was at work in both circumstances, it only transported a fraction of the heat that convection did.
Because convection is simply heat conduction in the presence of fluid motion, some scientists do not consider it to be a fundamental mechanism of heat transmission. They think of it as a type of thermal conduction called "conduction with fluid motion."
