Isochoric Process
An isochoric process involves a scenario in thermodynamics where the volume of a system remains constant while its pressure and temperature may change. This concept is key in understanding how certain systems, like a heated can of gas that doesn't expand, behave under specific conditions. For students studying for board exams and competitive exams like JEE and NEET, grasping the isochoric process is essential. This article simplifies the concept and includes a solved example to show how it applies practically, helping you understand and remember this important thermodynamic principle.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is the Isochoric Process?
- Key points in the Isochoric Process
- Solved Examples Based on the Isochoric Process
- Summary

What is the Isochoric Process?
A Thermodynamic process in which volume remains constant is known as the Isochoric Process.
In this process, P and T change keeping P constant. So Gay-Lussac’s law is obeyed in this process.
Key points in the Isochoric Process
- Its Equation of state is given as $\frac{P}{T}=$ constant
$
\text { or } \frac{P_1}{T_1}=\frac{P_2}{T_2}=\text { constant }
$
- P-V Indicator diagram for an isobaric process
Its PV graph has slope $=$ infinity (i.e $\frac{d P}{d V}=\infty$,
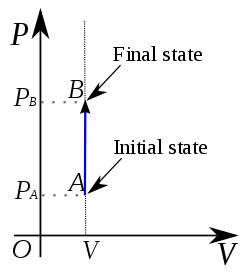
The above Graph represents an Isochoric increase in pressure at volume V.
The P-V diagram for this process is a line is parallel to the pressure line.
Specific heat of gas during the Isochoric process is given by
$C_V=\frac{f}{2} R$
The bulk modulus of elasticity during the Isochoric process is given by
$K=\frac{\Delta P}{-\Delta V / V}=\frac{\Delta P}{0}=\infty$
Work done in the Isochoric process-
$\begin{aligned} & \Delta W=P \Delta V \\ & \text { and as } \Delta V=0 \\ & \text { So } \Delta W=0\end{aligned}$
Internal energy in the Isochoric process
$\Delta U=n C_V \Delta T=n \frac{R}{(\gamma-1)} \Delta T$
Heat in the Isochoric process
From FLTD $\Delta Q=\Delta U+\Delta W$
But $\Delta W=0$
$
\text { So } \Delta Q=\Delta U=n C_V \Delta T=n \frac{R}{(\gamma-1)} \Delta T=\frac{P_f V_f-P_i V_i}{\gamma-1}
$
Example of Isochoric process
Heating of water in a pressure cooker (Valve closed)
Recommended Topic Video
Solved Examples Based on the Isochoric Process
Example 1: In an isochoric process, if $T_1=27^{\circ} \mathrm{C}$ and $T_2=127^{\circ} \mathrm{C}$ . Then $P_1 / P_2$ will be equal to:
1) 9/59
2) 2/3
3) 3/4
4) None of these
Solution:
Isochoric Process
When a Thermodynamic Process undergoes a physical change in such a way that its volume remains constant.
$\begin{aligned} & V=\text { constant } \\ & \therefore \frac{P_1}{T_1}=\frac{P_2}{T_2}\end{aligned}$
In the isochoric process, volume remains constant
At constant volume
$\begin{aligned} P & \propto T \\ \Rightarrow & \frac{P_1}{P_2}=\frac{T_1}{T_2}=\frac{273+27}{273+127}=\frac{300}{400}=\frac{3}{4}\end{aligned}$
Hence, the answer is the option 3.
Example 2: For a thermodynamic process $\mathrm{dP} / \mathrm{dV}=\infty$, it shows that the process is
1) Isothermal
2) Isochoric
3) Adiabatic
4) Isobaric
Solution:
Slope in P-V diagram for isochoric process
$
\frac{d P}{d V}=\infty
$
Since the line is parallel to the pressure line.
For isochoric process
$\mathrm{P}-\mathrm{V}$ diagram is
The slope of the P-V diagram
$\mathrm{dP} / \mathrm{dV}$
as $\mathrm{dV}=0$
so $\mathrm{dP} / \mathrm{dV}=\infty$
Hence, the answer is the option 2.
Example 3: A monatomic gas is going under an isochoric process, its specific heat will be
1) 5/2 R
2) 7/2 R
3) 5/3 R
4) 3/2 R
Solution:
For isochoric process
$
C_V=f / 2 R
$
$\mathrm{f}=3$ for monoatomic gas
$
\mathrm{Cv}=3 / 2 \mathrm{R}
$
Hence, the answer is the option 4.
Example 4: A cylinder with a fixed capacity of 67.2 lit. contains helium gas at STP. The amount of heat ( in J) needed to raise the temperature of the gas by:
$20^{\circ} \mathrm{C}$ is : [ Given that $R=8.31 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$ ]
1) 748
2) 374
3) 350
4) 700
Solution:
Specific heat for isochoric process
$
\begin{aligned}
& C_V=\frac{f}{2} R \\
& C_p-C_v=R
\end{aligned}
$
wherein
$f_{\text {is the degree of freedom }}$
1 mole at STP $\rightarrow 22.4$ it
So, 67.2 It $\rightarrow \frac{67.2}{22.4}$ mole
and the cylinder has with fixed capacity
So volume is constant means the isochoric process
$
\begin{aligned}
& Q=n C_V \Delta t=n\left(\frac{3 R}{2}\right) \Delta t \\
&=\left(\frac{67.2}{22.4}\right) \times \frac{3}{2} \times 8.314 \times 20 \\
& \Delta Q=748 . J
\end{aligned}
$
Hence, the answer is the option (1).
Example 5: A thermodynamic process undergoes a physical change in such a way that its volume remains constant, then the bulk modulus of elasticity for this process is
1) Zero
2) Infinite
3) can't say anything
4) one
Solution:
Bulk modulus of elasticity:
$
K=\frac{\Delta P}{-(\Delta V / V)}
$
For isochoric process
$
\begin{aligned}
& \Delta V=0 \\
& \text { So } \mathrm{K}=\frac{\Delta P}{0}=\infty
\end{aligned}
$
Hence, the answer is the option 2.
Summary
An isochoric process is a thermodynamic process that refers to the volume of a system remaining the same. However, the pressure and temperature of the system can change. However, no work is done by or on the system as the volume does not change. The system gets energy due to either addition or removal of it, and it appears as changes in internal energy and pressure. The temperature is directly proportional to the pressure for an ideal gas in an isochoric process.
Also Read
24 Dec'24 03:48 PM
02 Dec'24 12:39 AM
20 Nov'24 03:00 PM
16 Nov'24 12:51 AM
14 Nov'24 05:37 PM
12 Nov'24 01:14 PM
12 Nov'24 09:24 AM
25 Sep'24 05:44 PM
25 Sep'24 05:44 PM
25 Sep'24 05:43 PM