Magnetic Moment - Definition, Formula, Unit, Formation, Dipole Force Uses, FAQs
The magnetic moment is a basic physical property that indicates the amount and direction of a magnet or magnetic material. It applies to a variety of real-life applications, such as the working of electric motors and MRI machines, and some behaviours of Earth's magnetic field- the last helping compasses. Thus, an understanding of magnetic moments helps invent new technologies and efficient transformers for magnetic storage devices.

What is a Magnetic Moment?
The magnetic moment is also known as the magnetic dipole moment. It measures the tendency of alignment of an object in a magnetic field. When a magnet is placed in a magnetic field, it attempts to align with it. The magnet, which has a magnetic moment, is forced to a torque. This torque is used to define the magnetic moment. Magnetic moments can be found in magnets, current-carrying loops, atoms and molecules, subatomic particles, and so on.
In other words -The magnetic dipole moment, or strength of a magnetic dipole, can be regarded as a measure of a dipole's capacity to turn itself into alignment with a given external magnetic field. The magnetic dipole moment, also known as the magnetic moment, can then be defined as the greatest amount of torque induced by magnetic force on a dipole in vacuum per unit value of the surrounding magnetic field.
When considering a magnetic dipole as a current loop, the magnitude of the dipole moment is proportional to the current multiplied by the size of the enclosed region. The dipole moment's direction, which may be expressed mathematically as a vector, is perpendicular to the surface enclosed by the circular path of positive charge flow.
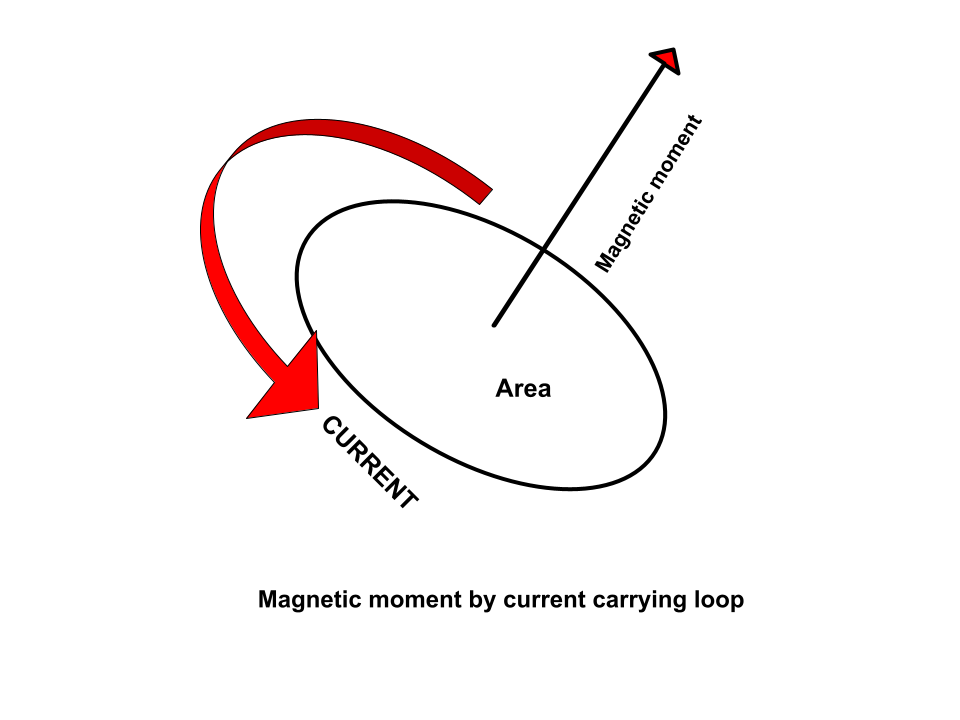
How is Magnetic Moment Created?
In general, the formation of the magnetic moment might have two origins. The first involves an electric and/or circular current with a current density distribution, whereas the second involves particles with their own angular momentum, known as spins.
How Is Magnetic Moment Measured?
Magnetic moments are typically measured by instruments known as magnetometers. However, not all magnetometers are aligned to measure the magnetic moment directly. Some of these devices measure magnetic fields only and from the measured magnetic field, the magnetic moment is measured.
Magnetic Moment Formula
The magnetic moment is expressed in
$$
\tau=m \times B
$$
Where,
1. m is the magnetic moment
2. $B$ is the magnetic field
3. $\mathbf{T}$ is the torque acting of dipole
Also, read
- NCERT Notes For All Subjects
- NCERT Exemplar Solutions for All Subjects
- NCERT Solutions for All Subjects
Unit of the Magnetic Moment
-
The si unit of magnetic moment is Amp-m2
-
We can write unit in terms of torque and magnetic field, torque is measured in Joule(J) and magnetic moment is measured in Tesla (T) hence the unit is $\mathrm{JT}^{-1}$
The following objects have a magnetic moment
-
Current carrying loop
-
Electromagnet
-
Atoms and molecules
-
Permanent magnet
| Related Topics |
Dipole Moment Uses
Dipole moment is used in various fields as follows:
-
Calculate the percentage of ionic character: The percentage of ionic character of covalent or ionic heteronuclear diatomic compounds is calculated using dipole moment data.
-
Differentiating between cis- and trans-isomers: the isomer with the greater dipole moment is the trans-isomer, and the isomer with the lower dipole moment is the cis-isomer.
-
The dipole moment is used to find the Bond angle.
-
It also assists in determining the size or structure of molecules, as well as the arrangement of chemical bonds inside them.
-
When it comes to determining the polar nature of a bond, the larger the dipole moment, the more polar the bond will be. Non-polar molecules have zero dipole moment, whereas polar molecules have a dipole moment.
-
When differentiating between ortho, meta, and para-isomers, the dipole moment of the para-isomer will be zero, while the dipole moment of the ortho-isomer will be higher than that of the meta-isomer.
Frequently Asked Questions (FAQs)
Magnetic moment is also known as magnetic dipole moment. It measures the tendency of alignment of an object in a magnetic field.
Magnetic moment is a vector because it has magnitude and direction.
The product of pole strength and the distance between the two poles is known as the magnetic dipole moment.