Neutrons Isotopes Isobars Isotones - Example, Difference, FAQs
Neutrons, isotopes, isobars and isotones are just common terminologies in the atomic structure because of the differences in atomic nuclei. An isotope is an atom that has the same atomic number with different neutrons whereas an isobar has the same mass number but differs in atomic number isotones are atoms that have the same number of neutrons and are different in protons.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What are isotones?
- What are Isotopes?
- What are Isobars?
- Difference Between Isotopes, Isobars and Isotones
What are isotones?
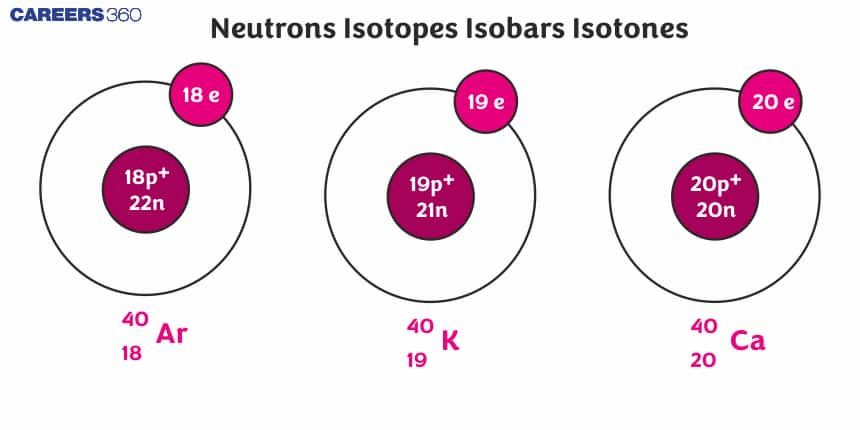
We can define isotones as if two species have the same number of neutrons they are isotones. Isotone's definition is “The nuclei having the same number of nucleons are called isotones”. For them, both the atomic number (Z) and mass number are different but the difference between (A-Z) is the same. Isotones examples are ₃Li⁷ and ₄Be⁸, ₁H³ and ₂He⁴, and ₁₁Ne²³ and ₁₂Mg²⁴.
Properties of Isotones
- Isotones are the nuclei having the same number of neutrons in the nucleus
- Atomic numbers and atomic masses of the Isotone species are different.
- The physical and chemical properties of isotonic species are also different. This is because these properties solely depend upon the number of electrons not on the number of neutrons.
What are Isotopes?
The atoms of an element whose nuclei have an identical number of protons but different numbers of neutrons are called isotopes of that element. In other words, different isotopes of an element have the same atomic number (Z) but a mass number (A). Because of the same atomic number, the isotopes of an element have the same place in the periodic table. almost every element has isotopes. krypton has 6 and 10 has 10 isotopes. isotopes of some elements are the following :
Hydrogen: ₁H¹,₁H², ₁H³
Oxygen: ₈O¹⁶,₈ O¹⁷, ₈O¹⁸
Neon: ₁₀Ne²⁰, ₁₀Ne²¹, ₁₀Ne²²
Chlorine: ₁₇Cl³⁵, ₁₇Cl³⁷
Uranium: ₉₂U²³⁵, ₉₂U²³⁸
Also, read
- NCERT Notes For All Subjects
- NCERT Solutions for All Subjects
- NCERT Exemplar Solutions for All Subjects
The hydrogen atom has three isotopes, each having atomic number 1 but their mass number are 1 2 and 3.₁H¹ nucleus has one Proton only, ₁H² nucleus has 1 proton and 1 neutron, ₁H³ nucleus has 1 proton and 2 neutrons. These elements are also known as an isotonic elements.
Properties of Isotopes
1. All isotopes of an element have the same number of electrons an atom has an equal number of electrons and protons. Therefore, the chemical properties of different isotopes of an element are the same.
2. The mass number( that is, the number of nucleons) of different isotopes of an element is different. Ha Hence, their physical properties are not the same.
3. Chemical properties are the same, two isotopes of the same element cannot be separated by any chemical process. To separate them, physical processes based on atomic mass, like gaseous diffusion are used.
4. Among isotopes of the same element, some may be stable and some radioactive. this is so because of the difference in their nuclear structure. For example, ₆C¹² is stable while ₆c¹⁴ is radioactive.
What are Isobars?
The nuclei have an identical no of nucleons, but different numbers of protons and different numbers of neutrons are called isobars. Their atomic number (Z) is different but the atomic number (A) is the same. Therefore they have different places in periodic numbers and also differ in chemical properties. Since in isobars, the numbers of fundamental particles are different, they differ in physical properties also. The nuclei of isobars belong to different elements. Some examples of isobars are ₁H³ and ₂He³, ₆C¹⁴ and ₇N¹⁴, ₈O¹and ₉F¹⁷.
Difference Between Isotopes, Isobars and Isotones
Isotopes | Isobars | Isotones |
The atoms of an element whose nuclei have the same number of protons but different numbers of neutrons are called isotopes. | The nuclei which have an identical number of nucleons, but different numbers of protons and different numbers of neutrons are called isobars. | The nuclei having identical numbers of neutrons are called isotones. |
Their atomic number (Z) is the same but a mass number (A) is the same. | Their atomic number (Z) is different but a mass number (A) is the same. | Their atomic number (Z) and mass number (A) are different, but the value of (A - Z ) is the same. |
Almost all the isotopes have identical chemical and physical properties. | Isobars have different physical properties and chemical properties. | Isotones have different physical and chemical properties. |
E.g., ₁H¹,₁H², ₁H³ | E.g.,₁H³ and ₂He³ | E.g., ₃Li⁷ and ₄Be⁸, |
Frequently Asked Questions (FAQs)
The atomic number of uranium is 92 and the mass number of uranium is 238.
So, the number of neutrons in uranium is 238-92 = 146.
The nuclei having the same number of nucleons are called isotones. Examples of isotones are
₃Li⁷ and ₄Be⁸, ₁H³ and ₂He⁴.
The meaning of Ar in chemistry is “argon”. It is an inert gas element.
isotopes isobars isotones with examples
Isotopes: ₈O¹⁶,₈ O¹⁷, ₈O¹⁸, ₁₀Ne²⁰, ₁₀Ne²¹, ₁₀Ne²²
Isobars: ₆C¹⁴ and ₇N¹⁴, ₈O¹and ₉F¹⁷
Isotones: ₁H³ and ₂He⁴, and ₁₁Ne²³ and ₁₂Mg²⁴
Isobars: The nuclei have an identical no of nucleons, but different numbers of protons and different numbers of neutrons are called isobars.
Isotopes:- The atoms of an element whose nuclei have the identical number of
protons but a different numbers of neutrons are called isotopes of that element.
Also Read
28 Nov'24 01:14 AM
16 Nov'24 01:37 PM
14 Nov'24 05:50 PM
12 Nov'24 01:15 AM
24 Sep'24 04:35 PM

