What is Nuclear Fission - Definition, Difference, FAQs
In this article, we explain nuclear fission basics, fission and fusion definition, the energy released in fission, nuclear fission and fusion example, spontaneous fission, and the application of nuclear fission.
Note: Nuclear fission in Hindi is विखण्डन
Nuclear fission basics:
Atomic structure: An atom consists of electrons revolving around the nucleus which is present at the center. The nucleus of an atom consists of a proton, neutron. Electrically positive charges are protons, neutrons are electrically neutral. Protons and neutrons are together called nucleons.
The atomic number of an element is the number of protons present in the nucleus of an atom and it differentiates one element from another. The atomic number is denoted by Z. Elements can have the same number of protons but not the same number of neutrons. Elements having the same number of protons and a different number of neutrons are called isotopes. Isotopes have the same atomic number.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Nuclear fission basics:
- What is nuclear fission reaction?/write nuclear fission meaning.
- Application or uses of nuclear fission:
- Difference between nuclear fission and nuclear fusion:
The sum of protons and neutrons gives the mass number of an atom. The mass number is independent of electrons as its mass is negligible.
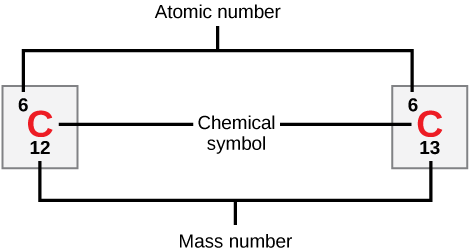
Figure 1 Representation of atomic number, chemical symbol, and mass number of isotopes of Carbon
As shown in the above figure, Carbon C has two stable isotopes having mass numbers 12 and 13. The atomic number is 6.
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
Binding energy:
A strong nuclear force binds the nuclei of an atom. The minimum energy by which the nucleus of an atom is divided into constituent protons and neutrons is called the binding energy. Binding energy is specific for different nuclei. The binding energy of the nucleon is much greater than the electrical forces present in between the electrons. The difference in the binding energy between the initial nucleus and the end product of nuclear reaction constitutes nuclear energy. Binding energy is also stated as the amount of energy released when a nucleon is disassembled from the nucleus.
What is nuclear fission reaction?/write nuclear fission meaning.
According to Einstein’s mass-energy relation,
Energy, E=mc2 where m is the mass and c (c=3x108 m/s2) is the speed of light.
Consider for m= 1 kilogram, energy E=1kg×(3×108)2
=1×3×108×3×108 Joules
=9×1016 Joules
For 1kg of mass, a huge amount of energy is released. Nuclei with higher binding energy per nucleon have a lower atomic weight per nucleon since mass and energy are comparable. The bombardment of heavy atoms with their neutrons is required in splitting those atoms. Uranium and plutonium are some of the heavy atoms.
The reaction in which the nucleus of an atom splits into two or more lighter nuclei with the release of energy is called nuclear fission.
The mass and energy of nuclear fission are represented as follows:
Consider the binding energy of the heavy nucleus be Bh and two fission products be B1 and B2.
Then the amount of energy released per fission, E=(B1+B2)-Bh
Amount of mass transferred to energy, E/c2 =[(B1+B2) -Bh]/c2
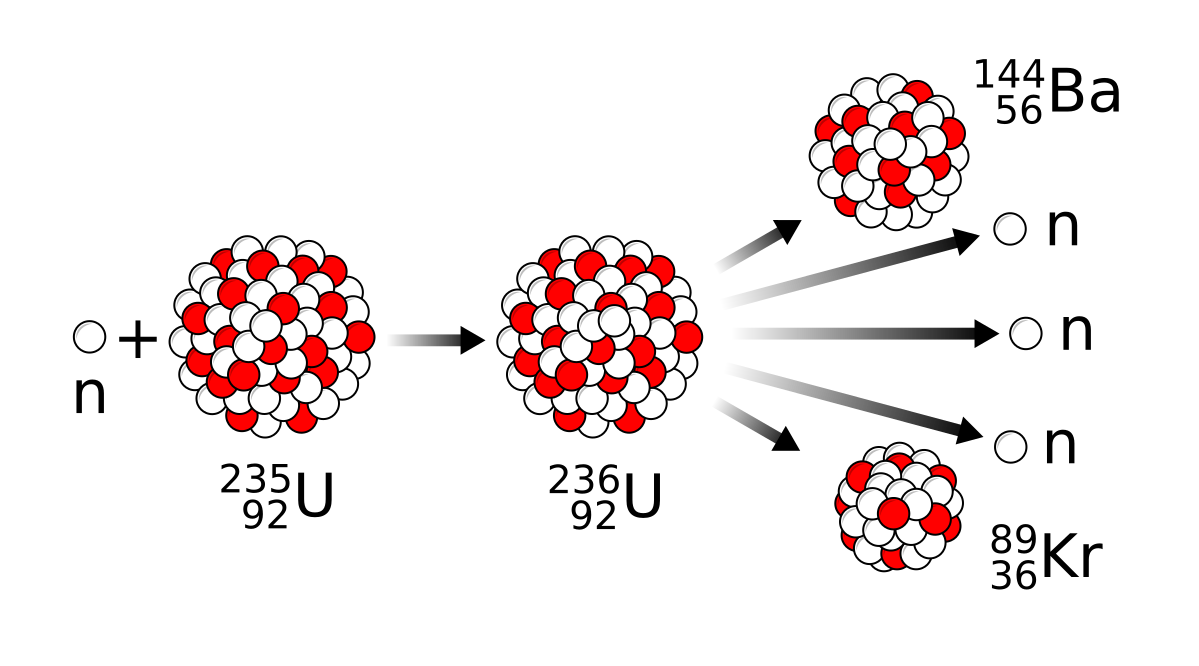
Figure 2 Nuclear fission reaction in U-235 atom
The above nuclear fission diagram shows the nuclear fission in Uranium-235. Here 235 is the mass number of uranium. In the first step, uranium-235 splits into uranium-236 by absorbing a neutron. Uranium-236 then undergoes fission and splits into two fragments. Nuclear fission results in further fragments.
To have a controlled chain reaction, the end product should trigger further fission. The chain reaction is the one in which additional fission occurs at least one further nucleus by the neutrons released in fission. Controlled nuclear fission is possible only when one nucleus hits the other uranium nucleus.
Nuclear reactors are the device used to control a nuclear fission reaction.
The uranium nuclear fission equation is given by,
23592U+n⟹23692U
23692U⟹9038Sr+14454Xe+2n+E
E is the fission energy released by the nucleus and n is the neutron.
Related Topics Link, |
Radioactive decay:
By radiation, if an unstable nucleus loses energy in a fission process then it is called radioactive decay.
Spontaneous fission:
If the unstable nuclei of heavier elements divide into two identical fragments then that radioactive decay is called spontaneous fission.
Application or uses of nuclear fission:
Nuclear fission is useful as follows:
- Nuclear fission reaction is widely applied in nuclear power plants.
- Nuclear fission reactions in Uranium and Plutonium are commonly used in nuclear power reactors.
- Nuclear fission is a principal source of heat and electricity.
- It supplies power for propelling submarines.
- Nuclear fission produces rare radioisotopes which have application in photographic devices.
Also Read:
- NCERT solutions for Class 12 Physics Chapter 13 Nuclei
- NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei
- NCERT notes Class 12 Physics Chapter 13 Nuclei
Difference between nuclear fission and nuclear fusion:
Nuclear fission and nuclear fusion are differed by the following factors:
- Nuclear fission is a reaction in which the nucleus of an atom breaks into lighter nuclei. Whereas nuclear fusion is a reaction in which two or more lighter nuclei bind together to form a heavier nucleus.
- Nuclear fission requires less energy to break an atom while nuclear fusion requires more energy to bind the nuclei.
- At the end of the reaction, an enormous amount of energy is released in nuclear fusion whereas nuclear fission releases less energy.
- Fission reaction is not visible naturally but fusion is observed in stars and the sun.
- Nuclear fission is used in nuclear power plants and nuclear fusion is applied in small-scale fusion reactors.
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes:
Frequently Asked Questions (FAQs)
Splitting into two or more divisions is called fission.
Nuclear means the concept relating to the nucleus of an atom.
Nuclear fission means the splitting of atomic nuclei into smaller units whereas nuclear fusion is the binding of nuclei together.
Example for nuclear fission: Uranium atom splits into xenon, iodine, barium, etc.
Example for nuclear fusion: A Hydrogen nucleus fuses to form helium.
Nuclear fission is explained by the liquid drop model.
Dr. Otto Hahn, Dr. Lise Meitner, and DR. Fritz Strassman are the scientist who discovered nuclear fission. Dr. Hahn is also called the “Father of nuclear chemistry”.
The energy released in nuclear fission averages about 200MeV for U-235.
In the chain reaction fission of Uranium-235, the nucleus splits into two by absorbing the neutron.
Splitting up a heavy nucleus into smaller nuclei when bombarded with neutrons having low energy is called nuclear fission.
Through a nuclear reaction, when the nucleus of an atom splits into lighter nuclei it is called nuclear fission.
Also Read
28 Nov'24 05:49 PM
28 Nov'24 10:14 AM
28 Nov'24 10:06 AM
08 Oct'24 03:32 PM
24 Sep'24 05:17 PM
29 Aug'24 09:52 AM

