explain friedel craft acylation
The Friedel-Crafts acylation reaction involves the addition of an acyl group to an aromatic ring. Typically, this is done by employing an acid chloride (R-(C=O)-Cl) and a Lewis acid catalyst such as AlCl 3 . In a Friedel-Crafts acylation reaction, the aromatic ring is transformed into a ketone. The reaction between benzene and an acyl chloride under these conditions is illustrated below.
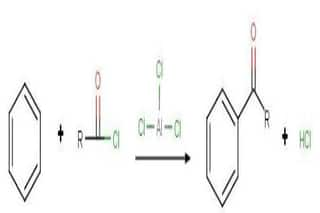
An acid anhydride can be used as an alternative to the acyl halide in Friedel-Crafts acylations. The halogen belonging to the acyl halide forms a complex with the Lewis acid, generating a highly electrophilic acylium ion, which has a general formula of RCO + and is stabilized by resonance.
Mechanism
Friedel-Crafts acylations proceed through a four-step mechanism.
Step 1
A reaction occurs between the Lewis acid catalyst (AlCl 3 ) and the acyl halide. A complex is formed and the acyl halide loses a halide ion, forming an acylium ion which is stabilized by resonance.
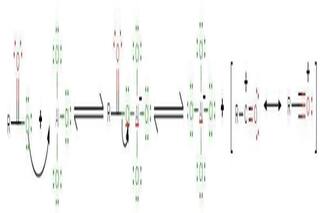
Step 2
The acylium ion (RCO + ) goes on to execute an electrophilic attack on the aromatic ring. The aromaticity of the ring is temporarily lost as a complex is formed.
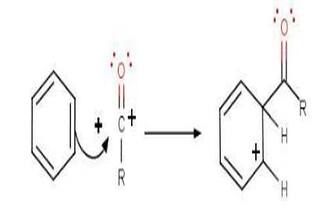
Step 3
The intermediate complex is now deprotonated, restoring the aromaticity to the ring. This proton attaches itself to a chloride ion (from the complexed Lewis acid), forming HCl. The AlCl 3 catalyst is now regenerated.
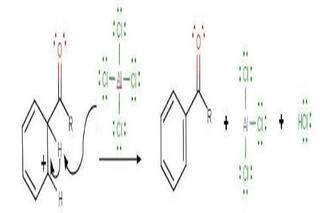
Step 4
The regenerated catalyst can now attack the carbonyl oxygen. Therefore, the ketone product must be liberated by adding water to the products formed in step 3. This step can be illustrated as follows.
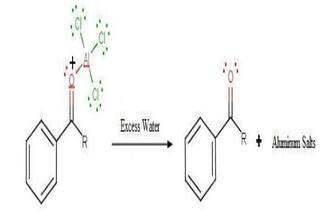
Thus, the required acyl benzene product is obtained via the Friedel-Crafts acylation reaction. Hope it'll help you to understand.

