62 Views
How to calculate normality of HCl?
Answer (1)
Formula for Normality:-
- Normality = ${\frac{[volume of solution in litres]}{[number of gramme equivalents]}}$
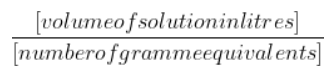
- Weight of the solute divided by the number of grammes equals the solute's equivalent weight is -1.
- 1 N = ${\frac{[Equivalent weight Volume (L)]}{Weight of Solute (gramme)}}$
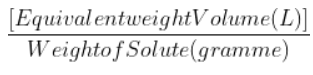
Related Questions
calculate molarityof 0.450 N HCl
632 Views
What is the formula of Normality?
24 Views
Shoolini University Admission...
Apply
NAAC A+ Grade | Ranked No.1 Private University in India (QS World University Rankings 2025)
Amity University Noida B.Tech...
Apply
Among Top 30 National Universities for Engineering (NIRF 2024) | 30+ Specializations | AI Powered Learning & State-of-the-Art Facilities
Graphic Era (Deemed to be Uni...
Apply
NAAC A+ Grade | Among top 100 universities of India (NIRF 2024) | 40 crore+ scholarships distributed



