Acid Anhydrides- Definition and Meaning of Acid anhydride, Synthesis, Chemical Properties
An acid is a substance that donates hydrogen ions to water. Anhydride is a chemical compound generated by discarding water from another compound. And we can also say that anhydride contains no water. Acid Anhydride is known as a non-metal oxide that gives an acidic solution when it reacted with water.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs
- Preparation Of Acid Anhydride
- Nomenclature Of Acid Anhydrides
- Examples Of Acid Anhydrides
- Properties Of Acid Anhydrides
- Uses Of Acid Anhydrides

In organic chemistry, acid anhydride consists of two acyl groups called the functional group combined with an oxygen atom. Here non-metals that are reacted with water are acid anhydrides and which are not reacted with water are not acid anhydrides. By this we can say that all non-metals are not acid anhydrides, non-metals react with water from acid and are acid anhydrides. Common acid anhydride in organic is carboxylic anhydride.
Preparation Of Acid Anhydride
To prepare acid anhydrides there are two general methods:
By Carboxylic acids
By Acyl chlorides
Acid anhydride by carboxylic acids:
To get anhydride, heat two carboxylic acids at a high temperature which means 800 degrees Centigrade. Then one water molecule will be got removed from the reaction.
Acid anhydride by acyl chlorides:
To get anhydride, on heating acyl chlorides with sodium salts in presence of pyridine.
Nomenclature Of Acid Anhydrides
Here, Nomenclature means naming things so the naming of acid anhydrides is very easy. So we simply replace the acid with anhydride and take the parent as it is. And this naming is the same for the International Union of Pure and Applied Chemistry (IUPAC) as well as the common name.
For example, if we take ethanoic acid we should change the name to anhydride so take the parent name which is ethanoic and replace acid with anhydride. Our final name is ethanoic anhydride.
As well as same for propanoic acid —> propanoic anhydride.
Examples Of Acid Anhydrides
Formation of carbon dioxide by carbonic acid, because carbon dioxide is an anhydride of carbonic acid.
$${{C}{O}_{2}}$$ + $${{H}_{2}{O}}$$ $$\to$$ $${{H}_{2}{C}{O}_{3}}$$
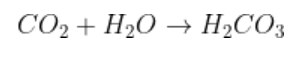
Formation of sulfur dioxide by sulfurous acid, because sulfur dioxide is an anhydride of sulfurous acid.
$${{S}{O}_{2}}$$ + $${{H}_{2}{O}}$$ $$\to$$ $${{H}_{2}{S}{O}_{3}}$$
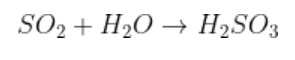
Properties Of Acid Anhydrides
Some acid anhydrides will get soluble in some solvents such as alcohol, and ether.
The boiling point is higher than their respective parent acids.
Acid Anhydrides are colorless, and also give a pungent smell.
These are less reactive compared to chlorides.
These will get hydrolyzed slowly with water.
They react with water to form carboxylic acids.
Acid anhydrides react with alcohols to form esters and with the esters to form amides.
Uses Of Acid Anhydrides
These are used in manufacturing things like industrial chemicals, explosives, perfumes, and pharmaceuticals.
Acid anhydrides are used as protecting groups.
Acid Anhydrides are used in the preparation of aspirin.
They are also used in the preparation of Esters through alcohol and Heroin by using morphine.
Frequently Asked Questions (FAQs)
Here, electron donation to one carbonyl group competes with the electron donation to the second carbonyl group. Thus, they are less stable and reactive in nature.
Sulfuric acid, Calcium oxide are some examples of acid anhydrides.
A compound acid that is formed by the removal of water is known as acid anhydride.
In liquid ethanoic anhydride, both dipole-dipole and van der Waals depression forces are present.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM