Aldol Condensation - Overview, Reaction, Types, Conclusion, FAQs
Aldol depletion occurs in α-hydrogen aldehydes with a mixed base providing β-hydroxy aldehydes called aldols. This reaction is more commonly known as aldol condensation. When a condensation reaction occurs between two different carbonyl chemicals it is called aldol condensation crossing.
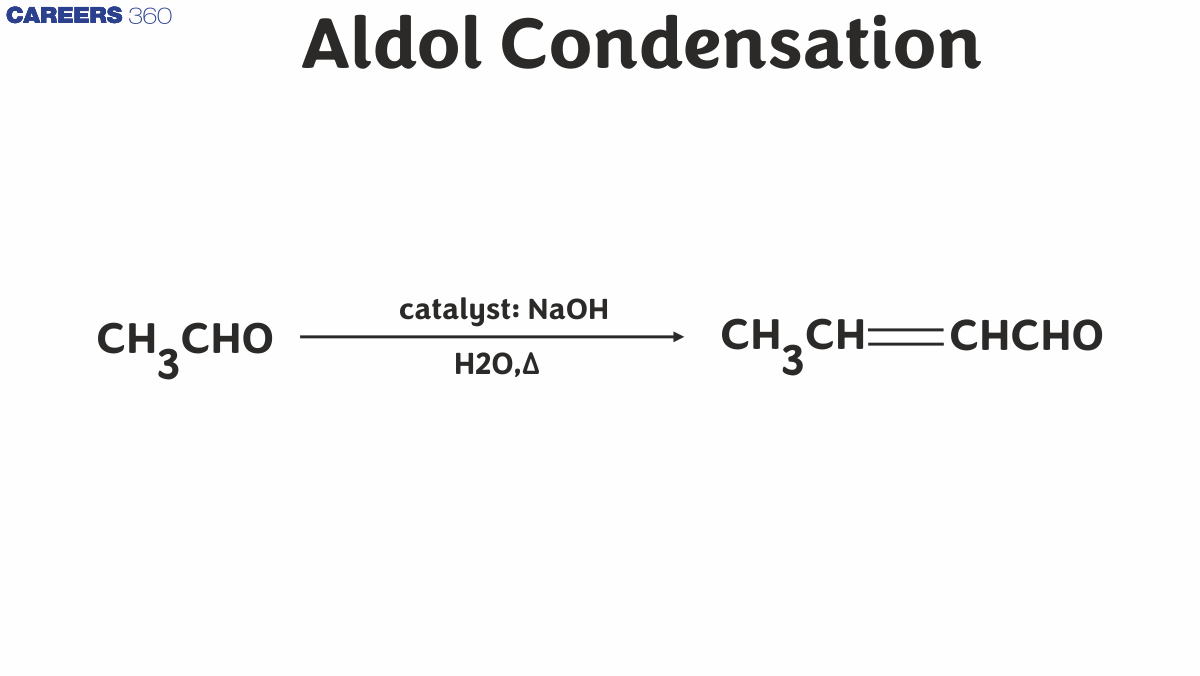
Aldol Condensation Reaction
Aldol condensation reaction: Aldol Condensation can be defined as a biological reaction where ions and a carbonyl compound are combined to form β-hydroxy ketone or β-hydroxy aldehyde, which is followed by dehydration to provide fused enone. Aldol Condensation plays an important role in the synthesis of organisms, creating a way to build carbon-carbon bonds.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Aldol Condensation Reaction
- Aldol Condensation Mechanism
- Crossed Aldol Condensation
- Types of Aldol Condensation
- Aldol Condensation of Acetone
- Acyloin Condensation
Aldol Condensation Mechanism
Step 1:
In a repetitive manner, the hydroxide ion absorbs the aldehyde.
Step 2:
Here Enolate ion 1 adds to the unadulterated aldehyde.
Step 3:
Alkoxide ion 2 is expressed in water.
Step 4:
A small amount of aldol is converted to an ion olate (4) by a hydroxide ion.
Step 5:
Here Enolate Ion (4) loses hydroxide ion.
Steps 1 to step 3 show the aldol reaction.
Crossed Aldol Condensation
The condensation reaction which occurs between two aldehyde molecules or ketone in protic solvents like water or alcohol triggers a cross-linked aldol reaction. When the condensate is between two different carbonyl compounds, it is called aldol condensation crossing. When both aldehydes contain alpha hydrogens, both can form carbanions and can act as carbanion receptors. A combination of four products was therefore made with a small amount of processing.
If one of the aldehydes does not contain alpha hydrogen then it can only act as a carbanion receptor. In that case, only two products were made. A typical substrate of an aldol reaction crosses a fragrant aldehyde, which has no alpha position. In addition, the dehydration of the first condensation product accelerates leading to the formation of α, β - an unrefined ketone and prevents the formation of retro-aldol from occurring.
Types of Aldol Condensation
It is important to differentiate aldol concentration from the various chemical reactions of carbonyl.
In the case of Perkin's reaction, the anolate made by the anhydride is odorless.
Claisen depletion contains two ester compounds.
Henry's reaction contains aliphatic nitro compound and aldehyde.
Dieckmann's depletion consists of 2 ester groups present in the same molecule, producing a rotating molecule.
In Jap - Maitland integration, water is removed by nucleophilic transfer.
Related Topics link |
Aldol concentration of Cyclohexanone
Frequent aldol maturation of cyclohexanone occurs as Intermolecular aldol condensation
Carbanion was built as an interior
It is a Nucleophilic reaction to that
Hydroxide acts as a base
There is also dehydration and provides a condensation product
Mechanism Response:
The aldol condensation reaction of cyclohexanone is a smart process of action.
Step 1: (Carbanion formation)
In the first step of the cyclohexanone aldol reaction, carbanion is formed as normal as a decrease in normal aldol. Base (Hydroxide ion) releases Alpha-Hydrogen for cyclohexanone. This molecule contains two Alpha-Carbons (Carbon presenting close to the active carbon-binding group) so here any Alpha-Hydrogen can be released. It leads to the formation of carbanions. It also deals with the enrichment of Enolate-carbanion
Step 2: (Electrophilic Center Attack)
In this step, the carbanion formed in the previous step invades the Electrophilic Center of another cyclohexanone molecule. That is why it is called Intermolecular aldol condensation
Step 3: (acid workup)
A small amount of acid is added here to convert the oxygen produced into its hydroxy form and the product produced is called Aldol's cyclohexanone product.
Step 4: (Dehydration)
The aldol product made of cyclohexanone enters the body at high temperatures and forms a condensation product (Water molecule is removed)
What is the aldol condensation product of cyclohexanone?
The product of Aldol condensation for cyclohexanone is [1,1′-bi (cyclohexylidene)] - 2-one
Related Topics link, |
Aldol Condensation of Acetone
Depletion of Aldol acetone in the presence of an acid catalyst gives diacetone alcohol (DAA) as an intermediate product, that further dehydrates to provide mesityl oxide.
By using active distillation (RD), one can improve the choice in the DAA, by continually removing it from the active area and thus suppressing the decompression response. The presence of water in the reaction organization has a significant impact on the internal response levels of each reaction. This water restriction effect can be used to advantage the improved selection of intermediate products.
The present study, through experiments and simulations, shows that the introduction of water in the RD can also increase the options targeted at the DAA. Groups of reaction kinetics in the presence of water are studied, and appropriate kinetic expression is proposed. In addition, batch tests were performed as well as continuous use of beverages to test their feasibility. Test results are defined with the help of an equity category model, and the required performance parameters are set according to the validated model.
Acyloin Condensation
Acyloin condensation is a degrading combination of two carboxylic esters that use metallic sodium to produce α-hydroxy ketone, also known as acyloin.
The reaction is most effective when the R is aliphatic and full. The reaction is performed on aprotic solvents with a boiling point, such as benzene and toluene in oxygen-deprived nitrogen (as oxygen molecules disrupt the reaction process and reduce yields). The use of solvents solvents leads to the Bouveault-Blanc reduction of esters different than the reduction. Depending on the size of the rings and the steric structures, but independent of the high reduction, acetyle inhibition of diesters prefers intramolecular rotation over intermolecular polymerization when using diesters.
To perform such reactions, it is suggested that the limits, in which there are ester groups, adsorbed, or are weak, at adjacent sites of sodium iron.
Therefore, the active end is not available for polymerization, thereby reducing the competitiveness of the circulation process. Diesters containing 10 or more carbohydrates make it easier to smuggle.
The mechanism of Acyloin Condensation consists of four steps:
(1) Oxidation ionization of both sodium atoms within the double bond of both ester molecules.
(2) Free mixing between two molecules of a homolytic ester derivative (Wurtz type coupling). Alkoxy degradation on both sides is possible, producing 1,2-diketone.
(3) Oxidative ionization of two sodium atoms in both diketone bonds. Sodium enodiolate is formed.
(4) Neutrality of water to form enodiol, which degrades acyloin.
Also check-
Frequently Asked Questions (FAQs)
Condition when aldehydes and ketones contain a single α-hydrogen treated with refined alkali that acts as a catalyst ,they form β-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol) respectively.
And this reaction is called as aldol condensation
Chloral CCl3CHO, has no α-hydrogen atom and hence does not undergo aldol condensation.
In learning aldol access one can follow the NCERT chemistry text-2 textbook for class 12. The chapter entitled 'Aldehydes, Ketones and Carboxylic acid' in this book contains this process.
The reaction is commonly used to produce solvents such as alcohol isophorone and diacetone. It works as an intermediate for perfume production. It is also used in pharmaceutical manufacturing.
Aldox process is an industrial variant of an aldol condensation reaction that is used for direct conversion of syngas as well as propene into 2-ethyl hexanol. This product is formed by hydroformylation of reactant to butyraldehyde, its subsequent aldol uptake into 2-ethyl hexenal, along with hydrogenation of this medium to 2-ethyl hexanol.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM