Alkoxymercuration Mechanism: Reaction, Example, Applications
An alkene (a molecule with a carbon-carbon double bond) reacts with alcohol in the presence of mercury acetate to produce an intermediate known as alkoxy mercury, which is then reduced with sodium borohydride to produce an ether.
This Story also Contains
- Oxymercuration Demercuration Reaction
- Oxymercuration Demercuration Mechanism
- Alkoxymercuration Mechanism Reaction Example
- Applications
In order to make ether under the appropriate circumstances, an alkene must be treated with too much alcohol while being in the presence of an acid catalyst. For instance, when 2-methylpropene and methanol are run over an acid catalyst, 2-methoxy-2-methylpropane is created.
The Alkoxymercuration-Demercuration Reduction is a challenging mechanism to comprehend. Students commonly forget the alcohol reagent and the alkyl group in the outcome, not because it is more complicated than oxymercuration.
Oxymercuration Demercuration Reaction
The alkene is transformed into alcohol through an additional process known as the oxymercuration-demercuration reaction. In this reaction, the alkene first interacts with mercury (II) acetate (Hg(OAc)2) in an aqueous solution of THF, and then sodium borohydride reduces the alkene (NaBH4)
Below is an illustration of an oxymercuration-demercuration reaction.
Isopropanol is made from propane.
The response is shown below.
C_3H_6\:\xrightarrow[2.NaBH_4]{1. Hg(OAc)_2,THF,H_2O}\:C_3H_8O
![]()
In this reaction, propene is combined with mercury(II) acetate in the presence of tetrahydrofuran, and the result is reduced to isopropanol using sodium borohydride.
The hydroxyl group is linked to the most substituted carbon atom in this reaction, and the hydrogen atom is joined to the least substituted carbon atom, in accordance with Markonikov's rule of regioselectivity.
Oxymercuration Demercuration Mechanism
In the alkoxymercuration process, an alcohol and an alkene are combined in the presence of a mercury salt, such as mercuric acetate, and the result is a demercuration step that yields ethers.
The process is comparable to the oxymercuration reaction, however, alcohol is used in place of water.
The electrophilic addition process is followed in this reaction. The primary distinction is that a mercurial ion bridge stabilises the intermediate carbocation, preventing it from rearranging. Electropositive charges are present in metals. The mercury, which has a partial positive charge, is the electrophile in the acetate complex.
The Alkoxymercuration-Demercuration Reduction is a challenging mechanism to comprehend. Students commonly forget the alcohol reagent and the alkyl group in the outcome, not because it is more complicated than oxymercuration.
In the first step of this reaction, the pi electrons attach to mercury, and the lone pair on mercury forms an ion bridge with the other vinyl carbon. The carbocation is stabilised by the mercury ion, preventing it from rearranging. The mercurial ion is created as a result of the loss of an acetate ion.
The second phase of this reaction involves the combination of an alcohol molecule with the most substituted carbon, which opens the mercurial ion bridge.
In the third step of this procedure, the addition product is neutralised by a proton transfer to an alcohol-solvent molecule.
The fourth stage of the reaction pathway involves reducing the organomercury intermediate with sodium borohydride at basic conditions.
The OR group is joined to the most substituted carbon and the H group is attached to the least substituted carbon in the reaction mechanism, which is based on Markovnikov's regioselectivity. The absence of strong acids and the fact that no separate carbocation intermediate forms make the reaction advantageous since it prevents carbocation rearrangements.
Alkoxymercuration Mechanism Reaction Example
After learning about the reaction, let's examine a concrete case to get a clearer visual. Consider the reaction of cyclohexene with ethanol and mercuric acetate as a model system. First, note that the reaction requires a carbon-carbon double bond, which is present in cyclohexene.
C_6H_{10}\:\xrightarrow{Hg(OAc)_2, CH_3CH_2OH, NaBH_4}\:(C_2H_5)_2O
![]()
Because we are using ethanol in this instance, we will eventually attach this molecule to our cyclohexene starting material and produce an ethyl ether. It's also important to remember that when the carbon-carbon double bond is broken, we also acquire a new carbon-hydrogen link in addition to the carbon-oxygen bond from the ether.
Applications
Oxymercuration is not just an alkene adding hydroxyl and mercury groups to water. Instead of waiting until a different reduction phase, the mercury in the carbon-mercury complex can spontaneously be replaced by hydrogen. In this way, mercury has the effect of acting as a Lewis acid catalyst. For instance, creating an enol by substituting an alkyne for alkene results in a ketone that has tautomerism. An ether results from replacing water with alcohol. Markovnikov's rule is followed in both scenarios.
When alcohol is present, the use of a vinyl ether enables the substitution of one alkoxy group (RO-) for another via an acetal intermediate. Under the right circumstances, an oxymercuration reaction between an allyl alcohol and a vinyl ether can produce
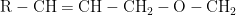 , which is appropriate for a Claisen rearrangement.
, which is appropriate for a Claisen rearrangement.
Frequently Asked Questions (FAQs)
The hydrogen atom is bonded to the carbon atom with the most hydrogen substituents, according to the Markovnikov rule.
Anti-Markovnikov Rule: The hydrogen atom is bonded to the carbon atom with the fewest hydrogen substituents by the Anti-Markovnikov rule.
Boron Hydride in Sodium Regarding "Demercuration" (The Second Step Of Oxymercuration-Demercuration) Also present in the oxymercuration reaction is NaBH4. In the second phase of the reaction, specifically, the C-Hg bond is broken and converted into a C-H bond using NaBH4.
A two-step, stereospecific process called alkoxymercuration-demercuration that proceeds in a Markovnikov fashion is used to make ethers (anti-addition).
Alkoxymercury is created when an alkene reacts with alcohol in the presence of mercury acetate. This intermediate is then reduced with sodium borohydride to produce an ether.
The Markovnikov rule indicates that the reagent's electron-rich element adds to the carbon atom with fewer hydrogen atoms bound to it in addition to reactions to asymmetrical alkenes. More hydrogen atoms are linked to the carbon atom by the electron-deficient element. In 1869, Vladimir Vasilyevich Markovnikov made the initial suggestion.
