Amines Identification: Primary, Secondary, and Tertiary Amines
Amines are organic compounds that have one or more hydrogen atoms from the ammonia molecule replaced with an alkyl or even an aryl group.
The Three Types Of Amines Are Primary, Secondary, And Tertiary.
Amines are also split into three groups, which are as follows:
Primary (1°)amine
Secondary (2°)amine
Tertiary (3°)amine
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- The Three Types Of Amines Are Primary, Secondary, And Tertiary.
- Which Method Is Used For The Determination Of Primary Amine?
- Identification Of Primary Secondary And Tertiary Amines
- Test Of Hinsberg
- Reaction To Hoffman Mustard Oil
- Conclusion
It is referred to as a secondary amine when it bonds with two carbon atoms, and as a tertiary amine when it bonds with three. All three types of amines—primary, secondary, and tertiary—display unique chemical characteristics as well as physical, discernible alterations. They are mostly employed for industrial and commercial purposes.
Amines typically have a lot of distinctive qualities, like their distinctive odours. These smells are typically those of fish or rotten eggs. Aliphatic amines have densities less than those of water and typically have stronger ammonia bonds than aromatic amines.
Which Method Is Used For The Determination Of Primary Amine?
The following procedure is used to identify the principal amines:
Explanation:
Diazotization is typically used to analyse aromatic compounds with molecules that contain an amino group.
It is frequently used to differentiate between primary aromatic amines and those that are aliphatic.
Diazo substances Diazo compounds are substances with generic formulas in which the diazo compound (-N=N) is linked to the groups on either side of the covalency.
Diazotization is the interaction of an organic acid, sodium nitrite, and a primary aromatic amine at a low temperature, preferably about 0°C, to produce a diazonium salt.
C_6H_5NH_2+2HCl+NaNO_2\longrightarrow\:C_6H_5N_2Cl+NaCl+2H_2O
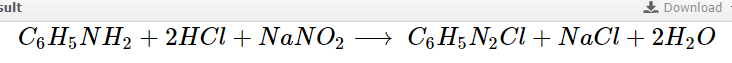
Aniline Benzenediazonium Chloride
Identification Of Primary Secondary And Tertiary Amines
Identifying the kind of Amines:
Amines are categorised as primary, secondary, or tertiary based on how many carbon atoms are directly connected to the nitrogen atom. In primary amines, nitrogen is connected to a single carbon.
In secondary amines, on the other hand, nitrogen is bonded to two carbons. The tertiary amines, in addition, have three carbon atoms that are covalently connected to nitrogen. All of these amines seem to have been grouped in the same manner as alcohols.
Alcohols, on the other hand, stand out due to the OH group's inclusion as a connection to the carbon atom. The carbons bonded to the nitrogen in amines are counted as carbons. The chemical characteristics and physical alterations that can be seen in secondary and tertiary amines are extremely diverse. The most typical application for them is in commercial and industrial settings.
Test Of Hinsberg
The Hinsberg test is a chemical procedure that is frequently used to identify primary, secondary, and tertiary amines. A Hinsberg reagent reacts with an amine when there is an aqueous alkali present. So, this is what the Hinsberg test refers to. The following observations are made following the reaction:
N-Ethylbenzenesulphonyl amide is typically generated when a primary amine reacts with a Hinsberg reagent, even though the reagent in question is benzene sulfonyl chloride. Due to the hydrogen that is bonded as a component of the nitrogen molecule, this is quite acidic. As a result, alkali can also dissolve this solution.
As a result, the Hinsberg test is useful for identifying primary, secondary, and tertiary amines. Amines are frequently used in many products, including several medicines and photos. Aside from this, the amine is also used to create pesticides and rocket fuel. Thus, in addition to their usual chemical usage, amines have a wide variety of other uses in the industry. Additionally, it serves heavy-duty military purposes including producing synthetic fibres required to make Kevlar, a vital component used to make helmets and bulletproof vests that protect soldiers in battle.
Reaction To Hoffman Mustard Oil
HgS is the cause of the black precipitate in the main amine Hofmann mustard oil reaction. It is a primary amine test.
Alkyl isothiocyanate, which has a mustard oil-like fragrance, is produced by primary amine.
By passing NHR in the presence of HgCl2, will get this equation
RNH_2+CS_2\longrightarrow S=C(SH)-NHR\rightarrow RNCS+HgS+2HCl
![]()
The reaction of Hofmann's mustard oil in secondary amine is not observed.
Conclusion
N-Ethylbenzenesulphonyl amide is frequently produced when a Hinsberg reagent combines with a primary amine, whereas the reagent used is known as benzene sulfonyl chloride. This is a very acidic solution because of the hydrogen connection to the nitrogen molecule. It dissolves in alkali as a result.
As a result, the Hinsberg approach can be used to distinguish between primary, secondary, and tertiary amine compounds. Amines can be used in a variety of fields, such as medicine and photography. This amine can be used to produce insecticides as well as rocket propellants. Amines are employed in a wide variety of industrial applications in addition to the usual chemical ones.
Frequently Asked Questions (FAQs)
An amine in which the amino group is directly linked to a non-carbonyl grouped carbon in any hybridization. X = any atom (often hydrogen) other than carbon.
The "e" ending of the longest chain's alkane name is changed to "amine" in the IUPAC system. When naming primary amines, the amino substituent -NH2 is used.
In addition to medicines and treatments, amines are used to create nylon and azo dyes. They are frequently employed in the creation of compounds for the filtration of water, and medicinal, and crop protection.
Amines are essential for the survival of life because they help to produce amino acids, which are the building blocks of proteins in living things. Amino acids are also used to make many vitamins. An essential amine called serotonin serves as one of the brain's main neurotransmitters.
At room temperature, amines, phosphines, and pyridines are typically high-boiling liquids or solids that are combustible but not particularly flammable. When amines burn, toxic NOx gases are produced.
Also Read
27 Sep'24 05:44 PM
27 Jan'24 11:41 PM
27 Jan'24 11:57 AM
26 Jan'24 10:37 PM
24 Nov'22 06:21 PM
18 Jul'22 03:38 PM
18 Jul'22 03:35 PM
