Arrhenius Acid: Definition, Examples, Uses, FAQs
The term "Arrhenius acid" refers to a material that contains a hydrogen atom and readily releases a hydrogen ion or proton when it is in contact with water. For instance, when hydrochloric acid dissolves in water, it produces the ions hydronium and chloride. Acetic acid (CH3COOH), which generates the acetate ion (CH3COO-) and the hydronium ion in its aqueous solution, functions similarly as an Arrhenius acid.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs
Corrosive poisons typically affect the eyes and lungs. Burns caused by acids or bases may potentially harm the cornea. The respiratory system is corroded by air contaminants such as sulphur oxides, nitrogen oxides, chlorine, and ammonia.
Arrhenius Acid
Svante Arrhenius, a Swedish scientist, first put forth the Arrhenius hypothesis of acids and bases in 1884. He proposed categorising some substances as bases or acids based on the ions they produced when combined with water.
The concentration of protons or H+ ions is raised by the presence of Arrhenius acid in the aqueous solution. Taking hydrochloric acid in water as an illustration. As will be discussed below, HCl undergoes a dissociation reaction to produce the ions H+ and Cl-. By producing the hydronium ion, the H+ ion concentration is raised.
Examples:
HCl _{(aq)} \longrightarrow H^+_{(aq)} + Cl^-_{(aq)}
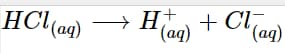
HCl_{ (aq)} + H_2O_{(l)} \longrightarrow H_3O^+_{(aq)} + Cl^–_{(aq)}
![]()
NHO_{3(aq)} + H_2O_{(l)} \longrightarrow H_3O^+_{(aq)} + No^-_3
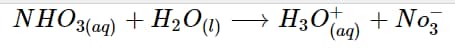
Arrhenius Base
A substrate that raises the number of hydroxide ions in an aqueous solution is known as an Arrhenius base. Highly soluble sodium hydroxide in water that separates into a sodium ion and hydroxide ion is an example of an Arrhenius base.
NaOH entirely dissolves to increase the concentration of hydroxide ions in an aqueous solution to produce sodium ions and hydroxide ions.
NaOH_{(aq)} \longrightarrow Na^+_{(aq)} + OH^–_{(aq)}
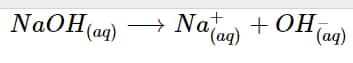
Strong Electrolytes From Arrhenius Acid
When Arrhenius sought to understand why some solutions could carry an electric current, he discovered that conductivity resulted from the presence of ions. He noticed that the chemicals HCl, HNO3, and H2SO4 behave as potent electrolytes when dissolved in water. Ionization reactions in water produced this.
These compounds are known as strong acids because they are powerful electrolytes that generate H+ ions. Ionization reactions in water produced this.
According to studies, when HCl, HNO3, and H2SO4 are added to water, almost all of the molecules dissolve to produce ions. This means that 100 H+ ions and 100 Cl- ions are created when 100 HCl molecules are dissolved in water. Since these compounds are strong electrolytes that generate H+ ions and are hence referred to as strong acids, there are almost no HCl molecules in an aqueous solution.
A Strong Acid
In an aqueous solution, strong acids totally ionise and raise the concentration of protons (H+).
HA \longleftrightarrow H^+ + A^–
![]()
Acid dissociation constant is the name of the equilibrium constant for an acid's dissociation (Ka). Strong acids have a very high Ka magnitude. As a result, the relationship between an acid's strength and its dissociation constant is linear (Ka).
Weak Acid
Weak acids do not produce hydrogen ions because of partial or incomplete dissociation; instead, they exist as an equilibrium mixture of undissociated acid and the ions released after partial dissociation. Compared to strong acids, hydrogen ions have a high pH value and a very low quantity of them. When compared to strong acids, weak acids have a lower acid dissociation constant.
Arrhenius Acid Examples
Hydrochloric acid, or HCl, is an excellent illustration of an Arrhenius acid. The hydrogen ion and chlorine ion are formed when it dissolves in water:
HCl _{(aq)} \longrightarrow H^+_{(aq)} + Cl^-_{(aq)}
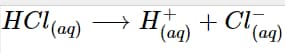
Because of the dissociation's effect on the number of hydrogen ions in the aqueous solution, it is regarded as an Arrhenius acid.
Nitric acid, hydrobromic acid, and sulfuric acid (
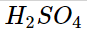 ) are further Arrhenius acids (
) are further Arrhenius acids (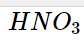 ).
).Sodium hydroxide (
 ) and potassium hydroxide are examples of Arrhenius bases (
) and potassium hydroxide are examples of Arrhenius bases ( ).
).
Problems With The Arrhenius theory
The Arrhenius theory only applies to aqueous solutions; for instance, it states that even if HCl gives an H+ ion to benzene, it is not an acid in aqueous solutions. Additionally, even though the amide ion deprotonates the ammonia, according to Arrhenius' definition, the sodium amide solution in liquid ammonia is not alkaline.
Frequently Asked Questions (FAQs)
It falls short of explaining the basic properties of non-hydrogenated compounds like NH3, CaO, MgO, and others, as well as the acidic properties of non-hydrogenated molecules like CO2, SO2, and SO3.
Aqueous media contain substances with acid-base properties. Acids and bases can be neutralised and hydrolyzed to varying degrees of strength.
An Arrhenius acid increases the concentration of hydrogen (H+) ions in an aqueous solution, whereas an Arrhenius base increases the concentration of hydroxide (OH-) ions. As a result, in Arrhenius acid-base reactions, the interaction between an acid and a base is a neutralisation reaction.
By determining if an acid or base produces hydrogen ions or H+ ions, and an acid or base produces hydroxide ions, or OH- ions, the Arrhenius hypothesis for acids or bases only applies if the substance is dissolved in water or an aqueous solution. Any solution, not only aqueous ones, should have the same characteristics as an acid or base. Additionally, the Arrhenius theory is limited to aqueous solutions exclusively.
Strong bases are those that totally separate into the cation and OH- in water (hydroxide ion). Group I (alkali metals) and Group II (alkaline earth) metal hydroxides are typically regarded as strong bases. These Arrhenius bases are typical ones.
Also Read
04 Oct'24 10:30 PM
04 Oct'24 10:16 PM
04 Oct'24 09:53 PM
04 Oct'24 09:25 PM
04 Oct'24 09:14 PM
30 Sep'24 11:47 AM
30 Sep'24 11:46 AM
30 Sep'24 11:44 AM
30 Sep'24 11:40 AM
30 Sep'24 08:50 AM
