Bomb Calorimeter
A bomb calorimeter is a device used for the determination of the heat of combustion of a given substance under observation. It operates in a rigid container, or a bomb, where the substance is burnt in the presence of excess oxygen. The heat that flows out due to the combustion process is then transferred to the surrounding water bath. The change in temperature of the surrounding water bath due to this transferred energy is used to determine the heat of the reaction. A bomb calorimeter is a device used to measure the heat of combustion of a substance. It consists of a strong container (the bomb) where the substance is burned in oxygen.
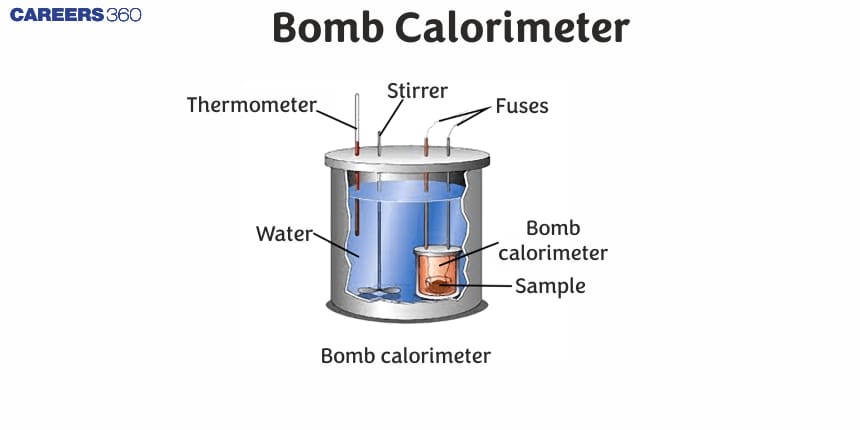
Bomb Calorimeter
In the laboratory, the heat released during combustion is measured in a bomb calorimeter.
It consists of an insulated vessel containing water and a rigid, constant-volume container (called a bomb) inside it.
The combustion process is carried out isochorically in the bomb and the heat released during combustion is trapped in the vessel and is used to raise the temperature of the calorimeter system.
The temperature change can be measured with the help of a thermometer and knowing the heat capacity of the system, the heat released due to combustion can be calculated.
Suppose T1 and T2 are initial and final temperatures and C is the heat capacity of the system, then
$\mathrm{Q}=\mathrm{C}\left(\mathrm{T}_2-\mathrm{T}_1\right)$
Now, since the combustion occurs in the rigid bomb, therefore the heat liberated is at constant volume and thus knowing the amount of substance undergoing combustion, the internal energy change during combustion can be calculated.
- If 1 mole of substance undergoes combustion then
$\mathrm{Q}=|\Delta \mathrm{E}|=\mathrm{C}\left(\mathrm{T}_2-\mathrm{T}_1\right)$
- If x g of substance (molar mass M) undergoes combustion then
$\mathrm{Q}=|\Delta \mathrm{E}| \times \frac{\mathrm{w}}{\mathrm{M}}=\mathrm{C}\left(\mathrm{T}_2-\mathrm{T}_1\right)$
Once, the value of $\Delta \mathrm{E}$ is calculated, we can calculate the $\Delta \mathrm{H}$ of the reaction using the following relation:
$\Delta \mathrm{H}=\Delta \mathrm{E}+\left(\Delta \mathrm{n}_{\mathrm{g}}\right) \mathrm{RT}$
The pictorial representation of a calorimeter system is given below

Recommended topic video on(Bomb Calorimeter)
Some Solved Examples
Example 1: The reaction of cyanamide, $\mathrm{NH}_2 \mathrm{CN}(\mathrm{s})$ with oxygen was run in a bomb calorimeter and $\Delta U$ was found to be -724.24kJmol-1 $\Delta H_{298}$ for the reaction
$\mathrm{NH}_2 \mathrm{CN} N_{(S)}+\frac{3}{2} \mathrm{O}_{2(g)} \rightarrow \mathrm{N}_{2(g)}+\mathrm{O}_{2(g)}+\mathrm{H}_2 \mathrm{O}_{(l)}$
is _______kJ. (Rounded off to the nearest integer)
[Assume ideal gases and R =8.314J mol-1 K-1]
1) -741
2)535
3)554
4)155
Solution
$\mathrm{NH}_2 \mathrm{CN}_{(\mathrm{S})}+\frac{3}{2} \mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{N}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})}+\mathrm{H}_2 \mathrm{O}_{(\mathrm{l})}$
$\begin{aligned} \Delta n_g & =\text { product }- \text { reactant } \\ \Delta n_g & =(1+1)-\frac{3}{2}=\frac{1}{2}\end{aligned}$
$
\begin{aligned}
& \Delta \mathrm{H}=\Delta \mathrm{U}+\Delta \mathrm{n}_{\mathrm{g}} \mathrm{RT} \\
& \Delta \mathrm{H}=-742.24+\frac{1}{2} \times \frac{8.314 \times 298}{1000} \\
& \Delta \mathrm{H}=-742.24+1.24 \\
& \Delta \mathrm{H}=-741 \mathrm{~kJ} / \mathrm{mol}
\end{aligned}
$
Magnitude of $\Delta \mathrm{H}=741$
Hence, the answer is ($\Delta H=741$).
Example 2: The heat of Combustion of ethanol into Carbon dioxide and water is at constant pressure. The heat evolved (in cal) at constant volume and $27^{\circ} \mathrm{C}$ ( if all gases behave ideally ) is ___________
$R=2$ calmol $^{-1} K^{-1}$
1) -326400
2)-56687
3)-56887
4)2152
Solution
The reaction occurs as follows:
$\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+3 \mathrm{H}_2 \mathrm{O}$
$\Delta$H = -327kcal
$\Delta$n = 1
Now,
$\Delta$H = $\Delta$U + $\Delta$nRT
$\Delta$U = $\Delta$H - $\Delta$nRT
$\Delta \mathrm{U}=-327+\frac{1 \times 2 \times 300}{1000}$
$\Delta$U = -326.4kcal
Hence, the answer is (-326.4kcal).
Example 3: For complete combustion of methanol
$\mathrm{CH}_3 \mathrm{OH}(\mathrm{l})+\frac{3}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
the amount of heat produced as measured by the bomb calorimeter is$726 \mathrm{~kJ} \mathrm{~mol}^{-1}$ at $27^{\circ} \mathrm{C}$ The enthalpy of combustion for the reaction, where x is _____________.(Nearest integer)
(Given : $\left.\mathrm{R}=8.3 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\right)$ )
1) 727
2)125
3)598
4)458
Solution
$\mathrm{CH}_3 \mathrm{OH}(\ell)+\frac{3}{2} \mathrm{O}_2(\mathrm{~g}) \longrightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\ell)$
Given, $\Delta \mathrm{U}=-726 \mathrm{KJ} / \mathrm{mol}$
$\mathrm{T}=27^{\circ} \mathrm{C}=300 \mathrm{~K}$
$\mathrm{R}=8.3 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}$
$\Delta n_g=1-\frac{3}{2}=-\frac{1}{2}$
Now, the relation between $\Delta \mathrm{U}$ and $\Delta \mathrm{H}$ is given as
$\Delta \mathrm{H}=\Delta \mathrm{U}+\left(\Delta \mathrm{n}_{\mathrm{g}}\right) \mathrm{RT}$
$\Rightarrow \Delta \mathrm{H}=\left(-726-\frac{1}{2} \times \frac{8.3 \times 300}{1000}\right) \mathrm{kJmol}^{-1}$
$\Rightarrow \Delta \mathrm{H}=-727.2 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Value of x is 727 .
Hence, the answer is (727).
Example 4: A gas (Molar mass $=280 \mathrm{~g} \mathrm{~mol}^{-1}$) was burnt in excess O2 in a constant volume calorimeter and during combustion, the temperature of the calorimeter increased. If the heat capacity of the calorimeter is and enthalpy of combustion of gas is $9 \mathrm{~kJ} \mathrm{~mol}^{-1}$ then the amount of gas burnt is_____________ g. (Nearest Integer)
1) 35
2)54
3)45
4)78
Solution
We know,
$
\begin{aligned}
& c_m=\frac{C}{n}=\frac{\text { Heat capacity }}{\text { mole }}\end{aligned}$
and $\Delta \mathrm{H}=\mathrm{C}_{\mathrm{P}} \Delta \mathrm{T}=\frac{\mathrm{C}}{\mathrm{n}} \Delta \mathrm{T}$
So, $g=\frac{2.5}{n} \times(258.45-298)$
$\mathrm{n}=\frac{2.5}{8} \times 0.45$
$\frac{\mathrm{w}}{280}=\frac{2.5}{9} \times 0.45 \Rightarrow \mathrm{w}=35 \mathrm{~g}$
Ans = 35.
Hence, the answer is (35).
Example 5: A sample of liquid in a thermally insulated container (a calorimeter) is stirred for 2 hr. by a mechanical linkage to a motor in the surrounding, for this process :
1)w < 0; q = 0; $\Delta$U = 0
2)w > 0; q > 0; $\Delta$U > 0
3)w < 0; q > 0; $\Delta$U = 0
4) w > 0; q = 0; $\Delta$U > 0
Solution
Thermodynamical Process -
The method or way by which we can change one Thermodynamic state to another state.
-wherein
Isothermal, Isobaric, Isochoric, Adiabatic
Work (W) -
Any type of energy transfer between the system & surroundings which is not due to temperature difference is known as work.
wherein
It is considered an ordered form of energy.
A sample of liquid in a thermally insulated container (a calorimeter ) is stirred for 2 hr. by a mechanical linkage to a motor in the surrounding, for this process: (d) w >0; q = 0; U > 0
Hence, the answer is the option (4).
Example 6: In a constant volume calorimeter,2.5g of gas with molar mass $28 \mathrm{~g} \mathrm{~mol}^{-1}$ was burnt in excess oxygen at 300k. The temperature of the calorimeter was found to increase from 300k to 300.5k due to the combustion process. Given that the Heat capacity of the calorimeter is $205 \mathrm{~kJ} \mathrm{~K}^{-1}$, the numerical value for the enthalpy of combustion of the gas in $k J \mathrm{~mol}^{-1}$ is:
1)$9 \mathrm{~kJ} \mathrm{~mol}^{-1}$
2)$11.6 \mathrm{~kJ} \mathrm{~mol}^{-1}$
3)$13.6 \mathrm{~kJ} \mathrm{~mol}^{-1}$
4) $12.6 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Solution
As we have learned,
Bomb Calorimeter -
In the laboratory, it is measured by using polythene on polystyrene bottles as follows.
Here 10 ml of each of acid and alkali having the same normality are taken in separate bottles and temperature is noted at regular intervals. When the constant temperature is achieved, the alkali solution is added to the acid solution. The mixture is stirred and the highest temperature is noted.
Suppose T1 and T2 are the initial and final temperatures here then
The rise in temperature = (T1-T2) K
Here specific heat capacity of the solution is assumed to be the same as that of water while heat capacity can be ignored as it is quite less than that of the solution.
$\mathrm{Q}=\mathrm{ms}\left(\mathrm{T}_2-\mathrm{T}_1\right)=\mathrm{ms} \Delta \mathrm{T}$
Given, $\Delta T=300.45-300=45 K$
The total energy released during combustion of 2.5 g gas
$=(\mathrm{ms}) \times \Delta \mathrm{T}$ $=2.5 \times 4.5$
So, heat released by 1 mole of gas combustion
$\begin{aligned} & =28 / 2.5 \times 1.125 \\ & =12.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\end{aligned}$
Hence, the answer is the option(4).
Summary
One of the key tools for chemical thermodynamics is bomb calorimetry, which measures the heat of combustion. Using a bomb calorimeter, after measuring the temperature change in the water bath jacketing the bomb, one can exactly calculate just how much energy was released during combustion. Such bomb calorimeter readings are an accurate source of information on the composition of energy and the efficiency of any fuels and compounds tested.