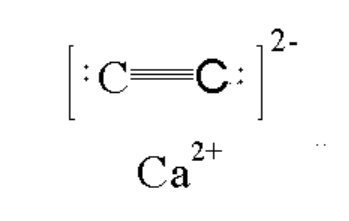
Calcium Carbide - Definition, Structure, Formula, Uses with FAQs
Have you ever wondered how a simple grayish solid, when dropped into water, can suddenly release a gas that burns with a bright, sooty flame? What makes this reaction so powerful that the gas produced is still used in welding and as a starting point for countless industrial chemicals? The answer of this question lies in this article on Calium carbide. Calcium Carbide $\left(\mathrm{CaC}_2\right)$ is an inorganic compound made up of calcium (Ca²⁺ ions) and the carbide ion (C₂²⁻). It is a greyish-black solid, industrially produced by heating lime (CaO) and coke (carbon) in an electric arc furnace at about $2000^{\circ} \mathrm{C}$
This Story also Contains
- Structure Of Calcium Carbide
- Carbide
- General Properties of Carbides
- Preparation of Carbides
- Different Types of Carbide
- Calcium Carbide
- Applications of Calcium Carbide
- Structure of Calcium Carbide
- Some Solved Examples
Here in this article, we will be discussing carbides, the uses of carbide, the formula of carbide, what is calcium carbide, the uses of calcium carbide, the structure of calcium carbide, the reaction of calcium carbide and water, the chemical formula of calcium carbide and aluminium carbide and some frequently asked questions related to carbides will be discussed here.
Structure Of Calcium Carbide
Calcium Carbide formula
The calcium carbide formula is $\mathrm{CaC}_2$.
Also read -
Carbide
Carbide is one of the most important terms which is used in both organic and inorganic chemistry. It is a chemical compound which consists of carbon and metallic or semi-metallic elements. It is the ionic or covalent bond that connects the carbide group to metallic or semi-metallic elements.
The formula of carbide ion is (C4-) and that of dicarbide ion is (C-22). It exists in ionic form and denotes that dicarbide ions are composed of two ‘C’ atoms. The formula of dicarbide (C-22) shows that it exists in dianionic form. Among various carbides, carbides of silicon, carbides of tungsten have a significant role because of their physical properties like strength, hardness and the ability to resist to chemical attacks at high temperatures.
Aluminium carbide (Al4C3), Beryllium carbide (Be2C), Calcium carbide ($\mathrm{CaC}_2$) etc. are some examples of carbide. Iron carbide or also called (Cementite) is an important component of steel and cast iron.
General Properties of Carbides
-
Carbides generally possess a very high melting point.
-
Carbides conduct heat and electricity.
-
Carbides are compounds with metallic lustre properties.
-
Alkali metal carbides are generally soft and transparent in a pure state. They are decomposed easily by water or acids and result in the formation of aliphatic hydrocarbons. They will not conduct electric current in solid-state.
-
All the coinage metal carbides are found to be coloured and explosive powders. Examples are Cu2C2 (dark brown), Ag2C2 (white), Au2C2 (yellow).
-
The carbides of alkaline earth metals are decomposed by dilute acids or water to form acetylene gas and they react with N2.
$\begin{aligned} & \mathrm{CaC}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{C}_2 \mathrm{H}_2 \\ & \mathrm{CaC}_2+\mathrm{N}_2 \rightarrow \mathrm{CaCN}_2+\mathrm{C}\end{aligned}$
Magnesium oxide is reduced to magnesium by such carbides. They also react with NH3 and Br2.
$3 \mathrm{MgO}+\mathrm{CaC}_2 \rightarrow 3 \mathrm{Mg}+\mathrm{CaO}+2 \mathrm{CO}$
$\mathrm{CaC}_2+4 \mathrm{NH}_3 \rightarrow \mathrm{CaCN}_2+\mathrm{NH}_4 \mathrm{CN}+4 \mathrm{H}_2$
$\mathrm{CaC}_2+4 \mathrm{Br}_2 \rightarrow \mathrm{CaBr}_2+\mathrm{C}_2 \mathrm{Br}_6$
-
Be2C has brick red colour and can scratch glass and quartz. Al4C3 is pale yellow in colour and it can be decomposed by water to produce methane. It can react with N2 to form Al5C3N and form alumina on reaction with oxygen.
$\mathrm{Al}_4 \mathrm{C}_3+6 \mathrm{O}_2 \rightarrow 2 \mathrm{Al}_2 \mathrm{O}_3+3 \mathrm{CO}_2$
Preparation of Carbides
-
Carbides can be synthesised by heating metal oxide with carbon.
$\mathrm{CaO}+3 \mathrm{C} \rightarrow \mathrm{CaC}_2+\mathrm{CO}$
-
It can also be manufactured from acetylene.
$\mathrm{C}_2 \mathrm{H}_2+2 \mathrm{Li} \rightarrow \mathrm{LiC}_2+\mathrm{H}_2$
| Related topics link, |
Different Types of Carbide
On the basis of the bond present between the carbide ion and the metal element, carbides are generally of the following types.
-
Ionic carbides- The combination of alkali or alkaline earth metals with carbide ions results in the formation of ionic carbides. The electrostatic attractive force binds these ions. There exists a huge electronegativity difference in this type of carbides. CaC2 is an example.
-
Covalent carbides- Low electropositive elements such as ‘Si’ and ‘B’ combines with carbide ions to form covalent carbides. In this type of carbides, there exists a low electronegativity difference. ‘SiC’ (Carborundum), B4C are examples.
-
Interstitial carbides- The combination of transition metals and carbide molecules forms interstitial carbides. In this type of carbide, carbide ions occupy the interstitial site of the metal lattice. TiC is an example.
-
Intermediate transition metal carbide – These types of carbides are also composed of transition metal and carbide ion and their size is found to be similar. Carbide of iron or known as cementite (Fe3C) is an example.
Uses of Carbides
-
Most of the carbides are found to be very hard. So they are used as abrasive tools, drilling and cutting tools.
-
They are used as reducing agents in metallurgy.
-
Al4C3 is used to synthesize methane whereas CaC2 is used for the manufacture of acetylene.
-
Carbides are also employed as furnace lining.
-
Boron carbide is generally used to cut diamond, to make filaments of lamps and also to drill holes in rocks.
-
The carbide of tungsten is nowadays used in making jewellery and also in the manufacture of various surgical tools.
Calcium Carbide
An important chemical compound with formula CaC2 is known as calcium carbide or calcium acetylide. One of its industrial use is that it is used for the synthesize of acetylene and calcium cyanamide.
In the pure state, it is found to be colourless, but anyway, the pieces of technical grade of CaC2 are grey or brown in colour and contain $80-85 \%$ of CaC2. The rest of it is composed of CaO, Ca3P2, CaS, Ca3N2, SiC etc.
CaC2 is mainly employed in the manufacture of acetylene gas and also to produce acetylene in carbide lamps. It is also used to synthesize various chemicals and fertilizers and is also involved in steel making.
Preparation
Calcium carbide is synthesized in an industrial scale by using an electric arc furnace by combining the mixture of coke and lime at $2200^{\circ} \mathrm{C}$. This is an endothermic reaction that requires 110 kilocalories per mole and very high temperature to expel CO.
$\mathrm{CaO}+3 \mathrm{C} \rightarrow \mathrm{CaC}_2+\mathrm{CO}$
The high temperature condition which is required for the manufacture of CaC2 cannot be gained through traditional combustion. Hence this reaction should be carried out in an electric furnace using graphite electrodes.
Also, students can refer,
Applications of Calcium Carbide
- Production of Acetylene: CaC2 reacts with water to form acetylene and calcium hydroxide.
$\mathrm{CaC}_{2(\mathrm{~S})}+2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{aq})} \rightarrow \mathrm{C}_2 \mathrm{H}_{2(\mathrm{~g})}+\mathrm{Ca}(\mathrm{OH})_{2(\mathrm{aq})}$
-
Production of calcium cyanamide: The reaction of CaC2 with N2 at a very high-temperature forms calcium cyanamide. It is generally known as nitrolime and is used as a fertilizer. It undergoes hydrolysis to form cyanamide H2NCN.
$\mathrm{CaC}_2+\mathrm{N}_2 \rightarrow \mathrm{CaCN}_2+\mathrm{C}$
-
Steel making: CaC2 is used in the desulfurization of iron and also as a fuel in steelmaking to extend the scrap ratio to liquid iron.
-
Deoxidizer: In ladle treatment it acts as a strong deoxidizer.
-
As a ripening agent: CaC2 is used as a source of acetylene gas which functions as an alternative ripening agent like ethylene.
-
CaC2 is also used to find out the moisture content of the soil.
-
CaC2 is nowadays sold as a mole repellent.
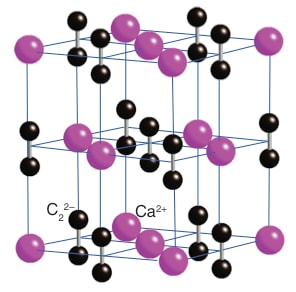
Structure of Calcium Carbide
The common crystalline form of CaC2 at room temperature is found to be a distorted rock salt structure in which $\mathrm{C}^{-2}{ }_2$ units are lying parallel. It can be shown as:
Also check-
Some Solved Examples
Question: In curing cement plaster, water is sprinkled from time to time. This helps in:
1) keeping it cool
2) (correct) developing interlocking needle-like crystals of hydrated silicates
3) Hydrating sand and gravel mixed with cement
4) converting sand into silicic acid.
Solution:
Water helps in the hydration of calcium aluminates and calcium silicates, which change into their colloidal gels by developing interlocking needle-like crystals of hydrated silicates.
The hydrolysis leads to the formation of calcium hydroxide which is useful in binding the particles of calcium silicate together.
Hence, the answer is option (2).
Question 2: When calcium carbide reacts with water, the gas evolved is:
A) Ethylene
B) Acetylene
C) Methane
D) Ethane
Solution:
$\mathrm{CaC}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{C}_2 \mathrm{H}_2+\mathrm{Ca}(\mathrm{OH})_2$
The gas produced is acetylene (C₂H₂).
Hence, the answer is option (2).
Question 3: Which of the following are true for $\mathrm{CaC}_2$?
1. Produced by heating lime and coke in an electric arc furnace.
2. Hydrolysis gives $\mathrm{C}_2 \mathrm{H}_2$.
3. $\mathrm{C}_2{ }^{2-}$ ion has a triple bond.
4. It is used to make calcium cyanamide.
A) 1, 2, 3
B) 2, 3, 4
C) 1, 2, 4
D) 1, 2, 3, 4
Solution:
All statements are correct. CaC₂ is a versatile industrial raw material.
Hence, the answer is option (4).
Question 4: When 6.4 g of CaC₂ reacts completely with water, the volume of acetylene obtained at STP (22.4 L/mol) is __ L.
1) 2.24L
2) 1.24L
3) 2.27L
4) 1.22L
Solution:
Molar mass $\mathrm{CaC}_2=64 \mathrm{~g} / \mathrm{mol}$.
Moles of $\mathrm{CaC}_2=6.4 / 64=0.1 \mathrm{~mol}$.
$1 \mathrm{~mol} \mathrm{CaC} \mathrm{C}_2 \rightarrow 1 \mathrm{~mol} \mathrm{C}_2 \mathrm{H}_2$.
So, $0.1 \mathrm{~mol} \rightarrow 0.1 \times 22.4=2.24 \mathrm{~L}$.
Hence, the answer is option (1).
Frequently Asked Questions (FAQs)
It is a chemical compound which consists of carbon and metallic or semi-metallic elements. It is the ionic or covalent bond that connects the carbide group to metallic or semi-metallic elements.
Carbides generally possess very high melting point, they conduct heat and electricity. Carbides are compounds with metallic lustre property.