Calcium Oxide - Overview, Structure, Formula, Examples, Uses, FAQs
Calcium oxide formula (CaO), sometimes known as quicklime or burnt lime, is a chemical substance that is frequently utilized. At room temperature, it is a white, caustic, alkaline, crystalline solid. The name "lime" refers to calcium-containing inorganic compounds that are mostly composed of carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron. The chemical formula of calcium oxide is CaO.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is lime water and give lime water chemical formula?
- Preparation of Calcium Oxide (quick lime)
- Structure of Calcium Oxide
- Properties of Calcium Oxide
- Chemical Properties of Calcium Oxide
- Uses of Calcium Oxide
Calcium oxide has a medium viscosity, a high surface tension, and a fast to medium rate of expansion and contraction. At ceramic temperatures, this substance is not volatile. Calcium oxide has a moderate effect on colour, unless it is used in big numbers, in which case it can bleach iron oxide. Free lime is calcium oxide that has not been processed and has not reacted in building materials such as cement.
What is lime water and give lime water chemical formula?
Calcium hydroxide is the chemical term for lime chemical formula, which has the formula Ca(OH)2. When water is introduced to lime, the following reaction occurs and hence calcium hydroxide (Ca(OH)2) is generated. Lime water formula or Quicklime formula(Quick lime formula) is Ca(OH)2. Chemical name of lime water is Calcium hydroxide.
CaO + H2O → Ca(OH)2
This reaction is very exothermic and results in the creation of large clouds of steam.
Preparation of Calcium Oxide (quick lime)
Calcium oxide can be made in a lime kiln by thermal breakdown of calcium carbonate (CaCO3; mineral calcite)-containing materials like limestone or seashells.
Calcination is the term for the process of making burnt lime. It is a method that begins by thermally decomposing the reactants at high temperatures but keeping the temperature below the melting point.
Calcium carbonate is calcined at temperatures ranging from 1070°C to 1270°C. In most cases, these reactions take place in a rotary kiln. Burnt lime and carbon dioxide are produced as a result of the process.
According to Le-Chatelier's principle, the carbon dioxide that is produced is immediately eliminated, delaying the reaction until the end of the reaction.
CaCO3 → CaO + CO2 (g)
Related Topics |
Structure of Calcium Oxide
One calcium cation (with a charge of +2) and one oxygen anion (with a charge of -2) make up a calcium oxide molecule. The structure is given below:
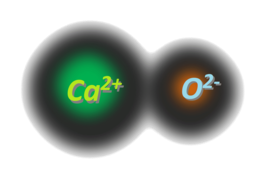
As a result, calcium oxide is an ionic compound that contains an ionic bond between calcium and oxygen.
Properties of Calcium Oxide
Quick lime is a white amorphous solid with a melting point of 2600°C.
It is highly stable, and even fusion cannot decompose it.
Calcium oxide crystallizes in cubic crystal lattice.
40 joules per mole kelvin is the normal molar entropy associated with calcium oxide.
When heated to temperatures above 2400°C, calcium oxide is known to create a bright glow.

(Calcium oxide powder)
Calcium Oxide | CaO |
Molar Mass | 56.0774 g/mol |
Density | 3.34 g/cm³ |
Boiling Point | 2850 °C |
Melting Point | 2572 °C |
Solubility | Soluble in glycerol and water. |
Chemical Properties of Calcium Oxide
When quick lime is hydrated, it becomes slaked lime or lime water. Lime become hot and cracks when water is added, resulting in a white powder. This is referred to as slaking of lime.
CaO + H2O → Ca (OH)2
Calcium oxide is a basic oxide that can form calcium salts when it reacts with acids.
CaO + H2SO4 → CaSO4 + H2O
With acidic oxides like silicon dioxide and phosphorus pentoxide, it creates silicates and phosphates. Because of its property, lime can be used as a flux in metallurgy to eliminate impurities.
CaO + SiO2 → CaSiO3
(Calcium silicate)
3CaO + P2O5 → Ca3(PO4)2
(calcium phosphate)
Also read -
Uses of Calcium Oxide
- It is used in the production of cement, paper, and high-grade steel, among other things.
In laboratories, lime is used as a reagent for dehydration, precipitation reactions, and other procedures.
It is the cheapest alkali accessible, and it is a key component in the production of caustic soda.
It is also used to remove hair from hides before they are tanned.
Used in insecticides and fungicides.
Used as a poultry feed preservative.
Calcium oxide is used in softening water and also in the recovery of ammonia.
Used as a filler to strengthen paper product.
Also check-
Frequently Asked Questions (FAQs)
Slaked lime is chemically calcium hydroxide (Ca(OH)2 also known as lime water and quick lime is calcium oxide (CaO).
Calcium causes brittleness in bones. Getting adequate calcium in your diet not only keeps your bones strong, but it may also help you avoid hypertension.
Quicklime basic flux and utilised in sugar refining as well as disinfectants and germicides.
Calcium oxide dissolves in glycerol and water.
Calcium oxide can harm the skin, nose, eyes, and respiratory system if it comes into contact with it.
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM