Carbylamine Reaction Mechanism - Overview, Example, Formula, Features, FAQs
Have you ever wondered how primary amines can be distinctly identified using a simple chemical test that produces an unpleasant odor? What happens when a primary amine reacts with chloroform and alcoholic potassium hydroxide, and why does this reaction fail for secondary and tertiary amines? You will find these answers by reading this article on the Carbylamine Reaction.
This Story also Contains
- Carbylamine Reaction
- Hofmann's Isocyanide Test
- Why does the given Carbylamine Reaction Mechanism not work in Secondary and Tertiary Amines?
- Amine's Features
- Isocyanide Test
- Isocyanide Formula
- Isocyanide Properties
- Isocyanide Synthesis
- Some Solved Examples
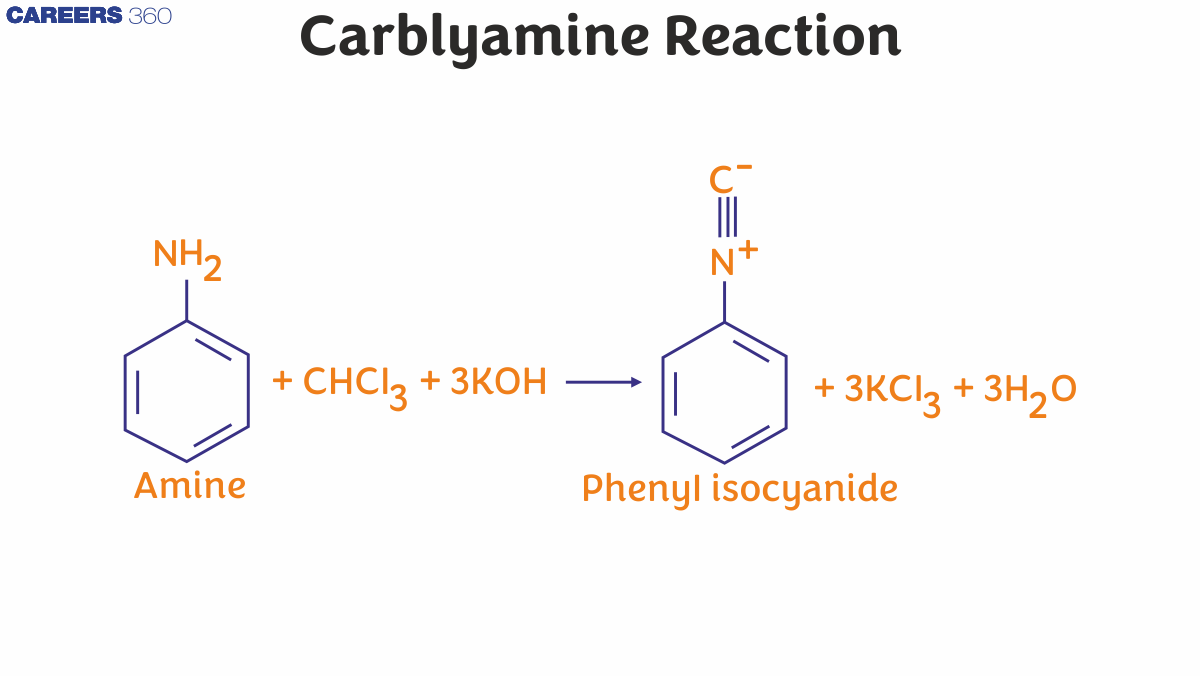
The Carbylamine Reaction Mechanisms are the combination of amines. Here, the intermediate effect is mediated by the dehydrohalogenation of the chloroform molecule. The intermediate form is called dichlorocarbene. Additionally, the carbylamine reaction mechanism is referred to as a combination of Hofmann isocyanide, which involves the reaction of chloroform, the main amine, and the basis for the synthesis of these isocyanides.
Carbylamine Reaction
The Carbylamine Reaction is a characteristic chemical test used to identify primary amines (–NH₂), both aliphatic and aromatic. In this reaction, a primary amine reacts with chloroform (CHCl₃) and alcoholic potassium hydroxide (KOH) on heating to form an isocyanide (carbylamine), which has a highly unpleasant, foul odor.
General Reaction
$\mathrm{R}-\mathrm{NH}_2+\mathrm{CHCl}_3+3 \mathrm{KOH} \rightarrow \mathrm{R}-\mathrm{NC}+3 \mathrm{KCl}+3 \mathrm{H}_2 \mathrm{O}$
Where $\mathbf{R}=$ alkyl or aryl group
R-NC = isocyanide (carbylamine)
Reaction Conditions
- Reagents: Chloroform $\left(\mathrm{CHCl}_3\right)$, alcoholic KOH
- Condition: Heating
- Medium: Alcoholic (ethanol)
Hofmann's Isocyanide Test
Because the Carbylamine Reaction Mechanism only works for the primary amines, we can use it as a chemical test for the availability of primary amines. When it is used as a test, the Carbylamine Reaction Mechanism is also called Hofmann's isocyanide test.
Here, the test material is heated with potassium hydroxide and chloroform.
In the presence of the first case of amine, the formation of isocyanide (carbylamine), which can be easily identified by its pungent odor, will occur. This Hofmann isocyanide test does not emit a bad odor either with second or higher amino acids because they have no carbylamine response.
Specificity of the Test
-
Positive: Primary aliphatic and aromatic amines
-
Negative: Secondary amines, tertiary amines, amides, and anilides
Reason: Only primary amines have the necessary hydrogen atoms on nitrogen required for the reaction.
Mechanism
-
Chloroform reacts with alcoholic KOH to form dichlorocarbene $\left(: \mathrm{CCl}_2\right)$.
-
The primary amine attacks the carbene.
-
Subsequent elimination leads to the formation of isocyanide.
Why does the given Carbylamine Reaction Mechanism not work in Secondary and Tertiary Amines?
The Carbylamine reaction (Hofmann’s isocyanide test) works only with primary amines because of structural and mechanistic requirements that secondary and tertiary amines do not fulfill.
Reason the Mechanism Fails in Secondary and Tertiary Amines
1. Absence of Required N–H Hydrogen Atoms
- Primary amines $\left(\mathrm{R}-\mathrm{NH}_2\right)$ have two hydrogen atoms attached to nitrogen.
- These N–H hydrogens are essential for: Formation of the reaction intermediate and Subsequent elimination steps leading to isocyanide (R–NC)
- Secondary amines $\left(\mathrm{R}_2-\mathrm{NH}\right)$ have only one N–H hydrogen
- Tertiary amines$\left(\mathrm{R}_3-\mathrm{N}\right)$ have no N–H hydrogen
Hence, the necessary elimination of HCl in the mechanism cannot occur.
2. Mechanism Requires Dehydrohalogenation
- After the primary amine attacks dichlorocarbene $\left(: \mathrm{CCl}_2\right)$, the intermediate must lose: Hydrogen from nitrogen and Chloride ion
- This dehydrohalogenation step is impossible in secondary and tertiary amines due to the lack of sufficient N–H bonds.
3. Steric Hindrance (Additional Factor)
- Secondary and especially tertiary amines are more sterically crowded.
- This reduces effective interaction with the highly reactive dichlorocarbene, further preventing the reaction.
Also read :
Why is Secondary Amine so much more basic than Primary and Tertiary Amines, and give the Carbylamine formula
There are some reasons why the secondary amine is the most basic compared to the first and second. Let's talk about this next.
Amines are found in ammonia, where hydrocarbon groups replace one, two, or all three hydrogen atoms.
When hydrogen is replaced by a single group, it is known as the main amine
[For example, methylamine (CH3-NH2)]; in two groups, known as secondary amine [For example, Dimethylamine (CH3-NH-CH3)]; and in three groups, it is known as high amine [For example, trimethylamine is CH3-N(CH3) -CH3].
Amines are essential in the environment for the reason that they hold fewer electrons in nitrogen. Therefore, they have a strong tendency to donate single electrons to electron receivers.
Amine's Features
The basis of amines depends on the substances given below.
Level of dissolved amine concentration, which includes a strong barrier to nitrogen groups.
Electronic structures (alkyl groups improve the base, while aryl groups reduce).
Because ammonium primary and secondary salts face soluble effects (due to hydrogen bonding) to a much greater extent compared to ammonium salts of high mineral water in water, the dissolving effects increase the electron density in amine nitrogen to a greater degree compared to that of the intervention effect of alkyl groups.
Although amine conjugate acid is very stable with a large number of Hydrogen compounds, its structure is less stable due to the abundance of its low electrons in the nitrogen atom. In contrast, electrons alone are not available for immediate display.
Therefore, the second amine is said to be more important compared to the first and higher because it is more stable than both methods, the ability of the conjugate acid that formed with it contains high electron density, and easy availability of reflective electrons, which equates to two groups, attached in it.
Isocyanide Test
Isocyanide, also called Isonitrile or Carbylamine, is a type of biological compound with a sub-atomic structure
R - N + ≡ C,
where R is a cohesive group driven by the expulsion of a hydrogen atom from a built environment.
Isocyanides are isomer nitriles; they were discovered in 1867 but have never achieved any major advantages.
They are usually extracted from essential oils for the treatment of chloroform and salt and are often found as small particles in the union of nitriles from iron cyanides and natural halogens. They smell amazing and amazing.
Isocyanide Formula
The formula Isocyanide (also called isonitriles) contains a nitrogen molecule attached to a carbon particle and an R (alkyl group), which has a replica structure that contains a triple bond, producing Cabanions and a fine nitrogen particle.
Isocyanide Properties
- Isocyanide suggests the following properties:
- Structure and Collaboration
- Spectroscopy
- The smell
- Poison
Now, we will understand each of these:
Isocyanide Synthesis
1. From Formamides
Isocyanides are commonly combined with dehydration. The reaction occurs in such a way that formamide is dehydrated with toluenesulfonyl chloride, phosphorus oxychloride, phosgene, diphosgene, or Burgess reagent before the base, such as triethylamine or pyridine.
The reaction is as follows:
$\mathrm{RNHC}(\mathrm{O}) \mathrm{H}+\mathrm{ArSO}_2 \mathrm{Cl}+2 \mathrm{C}_5 \mathrm{H}_5 \mathrm{~N} \rightarrow \mathrm{RNC}+\left[\mathrm{C}_5 \mathrm{H}_5 \mathrm{NH}\right]+\left[\mathrm{ArSO}_3\right]-+\left[\mathrm{C}_5 \mathrm{H}_5 \mathrm{NH}\right]+\mathrm{Cl}$
2. From Dichlorocarbon
In the reaction of carbylamine (the Alkyl isocyanide test is given by the Hofmann isocyanide test), the soluble base responds with chloroform to deliver dichlorocarbene.
Carbene then converts the four main into isocyanides.
Demonstrating the incorporation of tert-butyl isocyanide from tert-butylamine in the presence of a binding amount of catalyst transfer phase called benzyl triethylammonium chloride.
$\mathrm{Me}_3 \mathrm{CNH}_2+\mathrm{CHCl}_3(\mathrm{l})+3 \mathrm{NaOH}(\mathrm{aq}) \rightarrow \mathrm{Me}_3 \mathrm{CNC}+3 \mathrm{NaCl}(\mathrm{s})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
The above reaction occurs in the azo Dye test of amines. This test is also helpful in producing secondary amines.
3. Silver Cyanide Test
The first isocyanide, called allyl isocyanide, was prepared by the reaction of allyl iodide with silver cyanide. The reaction was as follows:
$\mathrm{RI}+\mathrm{AgCN} \rightarrow \mathrm{RNC}+\mathrm{AgI}$
4. By deduction
Isocyanides include the separation of oxazoles and benzoxazoles in the second place. The following compound of organolithium exists in the synthetic balance with 2-isocyano-phenolate, which can be trapped by an electrophile as an acidic chloride.
Also read -
Some Solved Examples
Question 1:
Compound ' A' reacts with nitrous acid to form p - p-nitroso N-N dimethyl aniline. Compound ' A ' is:
1) N - nitrosoaniline
2) p-nitroso N-methyl aniline
3) (correct) N - N dimethyl aniline
4) N - phenyl - N - methyl methanamine
Solution:
As we learn:
Carbylamine reaction -
The product is isocyanide & this reaction is used for the detection of primary amines.
- wherein
.png)
N - N dimethyl aniline
Hence, the answer is option (3).
Question 2: This compound does not respond to the carbylamine reaction:
1) Isopropylamine
2) (correct) Diethylamine
3) t-Butylamine
4) sec.Butylamine
Solution:
As we learn
carbylamine Reaction -
Product is isocyanide \& this reaction is used for the detection of primary amines.
- wherein
$\mathrm{RNH}_2+\mathrm{CHCl}_3+3 \mathrm{KOH} \rightarrow \mathrm{R}-\mathrm{NC}+3 \mathrm{KCl}+3 \mathrm{H}_2 \mathrm{O}$
an isocyanide (foul-smelling)
Hence, the answer is option (2).
Question 3: Which of the following species are involved in the carbylamine test?
(i) $R-N C$
(ii) $\mathrm{CHCl}_3$
(iii) $\mathrm{COCl}_2$
(iv) $\mathrm{NaNO}_2+\mathrm{HCl}$
1) (correct) (i) and (ii)
2) (iii) and (iv)
3) (ii) and (iii)
4) (ii) and (iv)
Solution:
Option (i) and (ii) are the correct answers.
Explanation: Amine, when it reacts with a mixture of $\mathrm{CHCl}_3$ and KOH, gives out alkyl isocyanate. This reaction is called a Carbylamine reaction.
Here,
$\mathrm{R}-\mathrm{NH}_2+\mathrm{CHCl}+3 \mathrm{KOH} \rightarrow \mathrm{RNC}+3 \mathrm{KCl}+3 \mathrm{H}_2 \mathrm{O}$
only RNC and $\mathrm{CHCl}_3$ are involved in carbylamine reaction.
Hence, the answer is option (1).
Question 4: The peptide that gives positive ceric ammonium nitrate and carbylamine tests is:
1) (correct) Ser-Lys
2) Gln-Asp
3) Lys-Asp
4) Asp-Gln
Solution:
As we have learnt,
Red coloration with Ceric ammonium nitrate confirms the presence of alcohols
Alcohol + Ceric Ammonium Nitrate → Red Solution
Foul smelling isocyanide obtained in the Carbylamine Reaction indicates the presence of Primary amines
$\mathrm{RNH}_2+\mathrm{CHCl}_3+3 \mathrm{KOH} \rightarrow \mathrm{R}-\mathrm{NC}+3 \mathrm{KCl}+3 \mathrm{H}_2 \mathrm{O}$
Thus,
Positive ceric ammonium nitrate -OH group
Positive carbylamine tests -NH2 group
(1) Ser-Lys ( Serine + Lysine )

Both amine as well as alcohol groups are present so it will give positive tests for both tests.
Hence, the answer is the option (1).
Practice More Question with the link given below
Frequently Asked Questions (FAQs)
Carbyl amines can be toxic and have a strong, often unpleasant odor. It is important to handle them in well-ventilated areas while wearing appropriate protective equipment (gloves, goggles) to prevent inhalation or skin contact.
The main amine (both fragrant and aliphatic), when heated with alcohol and chloroform KOH, produces isocyanides (otherwise called carbylamines). This is known as a Carbylamine Reaction Mechanism. Carbonyl Amines produce a bad odor. This reaction is given only the main amine and separates the main amine from other amine classes.
R-NH2 + CHCl3 + 3KOH → RNC (i.e., carbylamine) + 3KCl + 3H2O
Carbyl amines (also known as isocyanides) are organic compounds containing the functional group —N≡C—, which consists of a nitrogen atom triple-bonded to a carbon atom. They are often utilized in organic synthesis due to their unique reactivity.
Basically, there are 5 factors that affect the response rate, listed below.
- Pressure
- Temperature
- Focus focus
- Presence of catalyst
- The surface area of the molecule of the mixture
Carbyl amines participate in various reactions, including:
- Nucleophilic addition reactions
- Cyclization reactions
- Condensation reactions with carbonyl compounds
- Reactions with iodine (the so-called "carbyl amine reaction" leading to isocyanide synthesis)
Exposure to isocyanates can cause bronchitis and pneumonitis. The isocyanate reaction often includes cracking, chest tightness, airway, illness, vomiting, eye and skin tightness, abdominal pain, and loss of consciousness.
Excessive exposure to isocyanates can lead to aspiratory purification or "isocyanate asthma." When this happens, the symptoms improve when anger is released. In any case, severe asthma attacks occur in reconstituted openings, wherever they are, when the experience is short or with low levels of isocyanates, and can cause death.
Contact with the skin can cause inflammation and deterioration, which can lead to dermatitis. Wash hands with soap and water as soon as you come in contact. It is important to use hand washing and hand washing because isocyanates do not quickly dehydrate making it difficult to remove from the skin or clothing.
Common Isocyanates are found in car paint, froth pipes, sleeping cushions, car seats, froth protection, froth ties, under-cover cushioning, polyurethane and elastic.
A few professionals who can meet the isocyanates are car / truck shop designers, security makers, plastic cords and line manufacturers, and tire and furniture makers. However, they all use safety precautions to protect themselves from the dangers of isocyanide.
