Dispersed Phase and Dispersion Medium - Definition, Examples and FAQs
Two or more substances are homogeneously combined to form a solution. Those substances that get dissolved are referred to as solute and those substances in which solute is dissolved are solvents. For example, salt (solute) dissolved in water (solvent). The concentration of the solution is determined on the basis of the amount of solute dissolved in the solvent. Based on the amount of solute that is dissolved in the solvent, solutions can be further classified as saturated solutions, Unsaturated Solutions, and supersaturated solutions.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- What is a Supersaturated Solution?
- Examples of Supersaturated solution
- Supersaturation in Phase Change (Supersaturation Crystallization and Condensation)
- Applications of Supersaturated Solution
In the article, we cover the topic classification of supersaturated solution which is the sub-topic of chapter Solutions. it is important for board exams and JEE Mains Exam, NEET Exam, and other entrance exams.
What is a Supersaturated Solution?
Supersaturation meaning or supersaturated solution meaning is, in some cases, it is possible to prepare a solution that behaves unusually and contains more amount of solute than present in a saturated solution. Such a solution is referred to as a supersaturated solution or super solution.
A supersaturated solution definition or supersaturated solution definition chemistry is a solution containing more than the maximum amount of solute that can dissolve in solvent at a particular given temperature. A supersaturated solution possesses an unstable state; it could be made stable by separating the excess amount of solute dissolved in the solvent.
Related Topics Link, |
Examples of Supersaturated solution
Examples of supersaturated solutions include carbonated water (i.e. soda water), honey or sugar syrup used in confectionery, etc.
An example of supersaturation is shown by sodium thiosulfate (Na2S2O3). It can dissolve 50 g Na2S2O3 per 100 g of H2O at room temperature. Suppose, 70 g of Na2S2O3 crystal is dissolved in 100 g hot H2O and the solution is then cooled to room temperature. Then the additional 20 g of Na2S2O3 usually does not get precipitated.
The solution thus obtained is supersaturated and it is unstable. The recrystallization in a supersaturated solution can be performed by the addition of a small crystal of solute which is called a seed crystal. This process is defined as seeding in chemistry.
The nucleation site is provided by the seed crystal on which the extra dissolved crystals can begin to grow. The supersaturated solution of Na2S2O3 can be seeded by the addition of a Na2S2O3 crystal, in which the excess salt suddenly crystallizes and heat is liberated. After the crystals are settled and the temperature has cooled back to room temperature (25°C), the solution found above the crystal is saturated and it contains 50 g Na2S2O3. Recrystallization is a very fast process from a supersaturated solution.
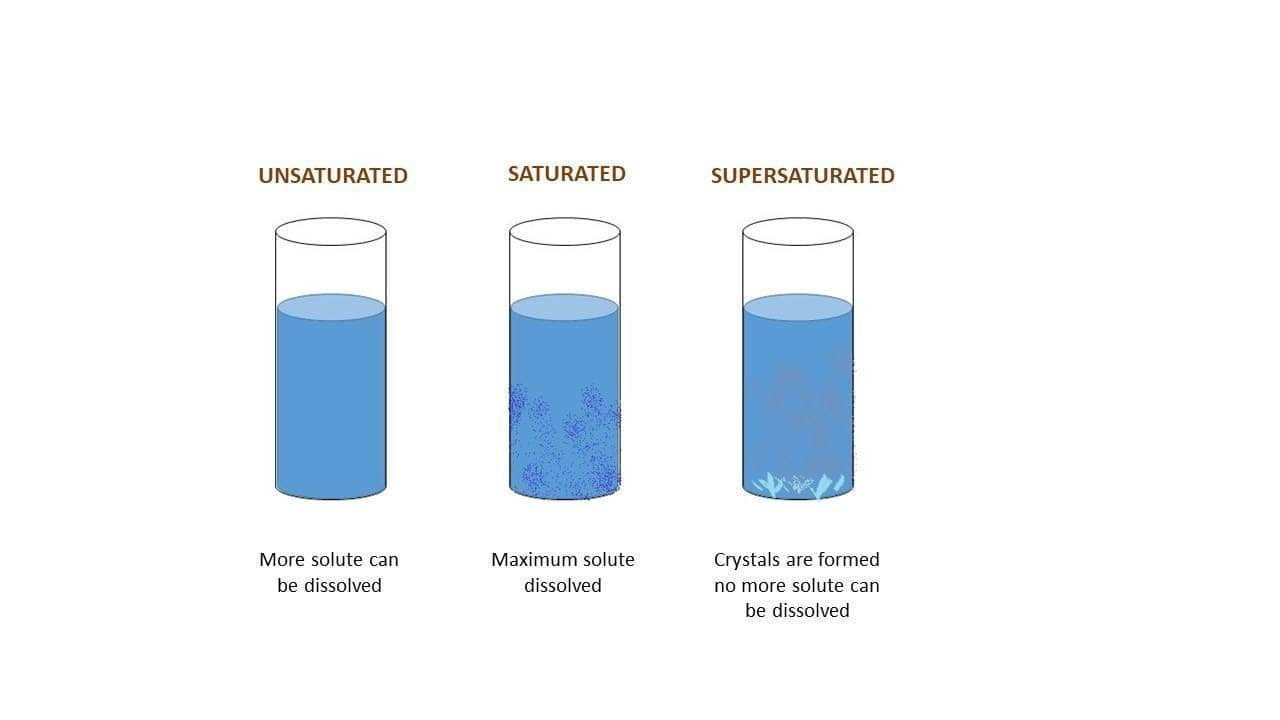
Supersaturation in Phase Change (Supersaturation Crystallization and Condensation)
- In each system, the physical and chemical processes of the vapor melt or the solution phase occurs by the formation of three-dimensional nuclei of a new phase and take place only during the supersaturated medium.
- The nuclei’s production is associated with a change in the free energy of the system. In the case of a homogeneous system, the new phase of the nuclei is not produced as soon as the system becomes supersaturated though thermodynamically, such a situation becomes possible.
- The system is found to be in a metastable equilibrium state, and it can remain in the same state without attaining the least or minimum free energy corresponding to the equilibrium state.
- In other words, in such cases, the nucleation of a new phase sets in after a time period, where the value depends on factors such as the pressure and temperature of the system, the presence of chemical phases varies from the increasing supersaturation level, and nucleating phase facilitates the nucleation process of the new phase.
- However, there is always a level of supersaturation when the new phase is instantaneously nucleated. It is referred to as the new phase precipitates.
- Such a supersaturation level signifies the upper limit of the metastable equilibrium state and describes the metastable width.
Also read -
Applications of Supersaturated Solution
- Supersaturation have practical applications in the field of pharmaceuticals. By preparing a supersaturated solution of a particular drug, it can be ingested in liquid form. The precipitation can be prevented by adding precipitation inhibitors. Drugs in such states are termed "supersaturating drug delivery services," or "SDDS." Oral consumption of a drug in this form is easy and helps in the measurement of very precise dosages.
- Marine ecologists use supersaturated solutions’ identification as a tool for the study of the activity of organisms and populations.
- Supersaturation is an important factor in the design of steam turbines.
- The study of supersaturation is also important for atmospheric studies. Actually, supersaturation of water is very common in the upper troposphere. This can be found using satellite data from the Atmospheric Infrared Sounder.
Also, check-
Frequently Asked Questions (FAQs)
A colloid is a substance wherein minute, microscopically dispersed insoluble debris of a substance is suspended in some other substance. The length of colloidal debris varies from 1-1000. A solution exists in a single-phase only, and no visible interface exists. Whereas in a colloid, unique phases, particularly the dispersed section and dispersion medium, exist. An interface among them may be observed.
Colloids are categorized on the basis of the interaction between the dispersed phase and the dispersion medium. They can be categorized into eight types-
Solid sol, Sol, Smoke, Gel (liquid dispersed in solid), Emulsion, Aerosol, Solid foam, Foam.
When light is allowed to pass through a true solution, it passes clearly through the solution whereas when passed through a colloidal solution, it scatters light in various directions making it observable. This is called the Tyndall effect.
The normal range of particles in a colloidal solution is 1- 1000 nm.
Dust is an aerosol type of colloid where a solid is suspended in a gas.
A supersaturated solution is a solution that contains more solute than what can normally be dissolved in a given amount of solvent at a specific temperature. This state is achieved by dissolving more solute than usual, often at elevated temperatures, and then allowing the solution to cool without any crystallization occurring.
To create a supersaturated solution, you typically heat a solvent and dissolve solute into it until no more solute can dissolve. Once the solution is saturated and no solid remains, you slowly cool the solution. If cooled carefully without disturbing it, the solution can remain supersaturated.
Common examples include rock candy, where sugar is dissolved in hot water and then slowly crystallizes as the solution cools. Another example is carbonated beverages, which can contain dissolved carbon dioxide under pressure, creating a supersaturated solution that releases gas bubbles when opened.
Also Read
19 Feb'25 04:59 PM
04 Nov'24 10:45 AM
07 Oct'24 12:46 PM
07 Oct'24 12:44 PM
04 Oct'24 06:04 PM
30 Sep'24 02:35 PM
30 Sep'24 02:28 PM
30 Sep'24 11:36 AM