Colloids
Colloids are one of those things that pervade our lives without us even realizing it, and they make a big difference. Think of a glass of milk. Picture the window with a streak-free shine from the new glass cleaner. Picture again that shampoo in your hand. They probably all have their unique properties because of the fascinating world of colloids that they belong to. A colloid is a mixture in which small particles of one substance are dispersed in, but do not chemically react with, a second substance to produce a homogeneous solution.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Types of Colloids
- Some solved examples
- Conclusion

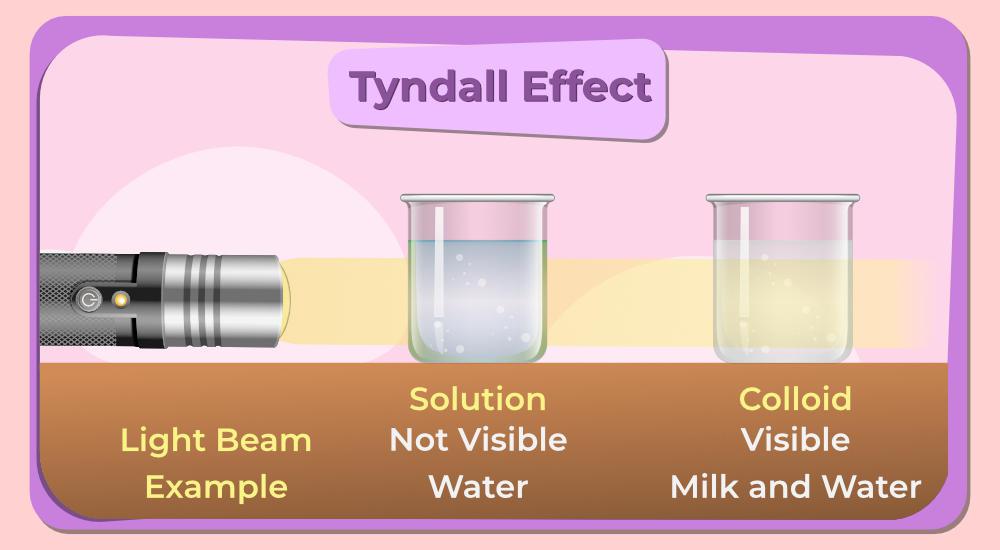
Colloids are heterogenous mixtures whereby one substance—the dispersed phase—is finely divided and uniformly dispersed within another substance, the continuous phase. The size of the particle in the colloid generally remains in the range of 1 to 1000 nm, larger than that found in solutions but smaller than in suspensions. This size range lends a few special characteristics to colloids, such as the Tyndall effect, whereby they scatter light and make the path of light visible through the colloidal dispersion.
Types of Colloids
Lyophilic and Lyophobic Colloids
Lyophilic Colloids: The solids that disperse in suitable liquids also show solubility. These are colloids, with a kind of affinity of the dispersing medium (solvent) towards a continuous phase. In simpler words, this could be gelatin and starch in water. These colloids are easily reversible in normal conditions and thus highly stable.
Lyophobic Colloids: There is no such good affinity of the dispersing medium (solvent). This means it has a bad affinity. For example, gold and silver in water. Such colloids are not easily reversible and are not very stable; therefore, they generally require stabilizing agents too.

Multimolecular and Macromolecular Colloids
Multimolecular Colloids
Definition: The colloids that are multimolecular have the dispersed molecules in the form of aggregates/clusters.
Particle Size: Size of the particle that is dispersed in multimolecular colloids is very small, generally less diameter less than 1 nm.
Examples
Gold sol: It contains the colloidal particles that are based on Au and stabilized by citrate ions and exists in water.
Hydrosols: These contain stabilized hydrophilic ions plus metallic oxides and sulfides that find existence as colloidal solutions.
Formation: These colloids are formed when the dispersed phase aggregates to the extent of forming particles of a very small size that gravity is overcome by the movement of the resultant molecules; and they always remain in suspension in the dispersion medium.
Stability: Such colloids are more stable than other colloids due to the presence of macromolecules in them. The macromolecular structure does not allow the particles to aggregate or coagulate.
Macromolecular Colloids:
Definition: Chemical compounds or mixtures in which large-sized molecules are dispersed are known as macromolecular colloids. These dispersed molecules may include polymers, proteins, synthetic polymers, etc. The substance in which the particles are dispersed is known as a solvent or dispersion medium.
Particle Size: The particle size of the substance, which is dispersed, is in the range of 1 to 1000nm and
Protein solutions: Albumin or gelatin in water are typical of this
Synthetic polymers: Polyvinyl alcohol (PVA) in a measured amount of water is in this class
Formation: The colloids result from the dispersion of large molecules or polymers in the medium, and such molecules remain in suspension due to their big sizes and the stabilizing influences of solvation with surface charge.
Stability: The macromolecular colloids are more stable than multimolecular colloids, with the large size of the dispersed phase and surface charges that might prevent rapid aggregation or precipitation.
Key differences
Size: Multimolecular colloids weigh very small, almost negligible quantities of even less than 1 nm. But macromolecular colloids are much in size, usually in the range 1 to 1000 nanometers.
Nature: One that is composed of small molecular aggregates; the other one that is composed of large molecules or polymers.
Colloidal stability: In comparison with the multimolecular colloids, the macromolecular colloids are relatively more stable because of the larger size of the particles, coupled with the possibility of their electrostatic repulsion or steric hindrance. The multimolecular colloids may easily combine into aggregates.
Associated Colloids
Associated colloids or micelles, form at concentrations above a certain level, known as CMC or critical micelle concentration. Soaps and detergents are familiar examples of associated colloids. At and above the CMC value, surfactants form agglomerates called micelles. The hydrophilic ends face the outer environment while the hydrophobic ends combine inside the micelle.
Cleaning Action of Soaps
Soaps are surfactants that consist of a long alkyl chain attached to a polar carboxylate group, which imparts hydrophilic properties to the compound. Much as birds realign themselves into an arrowhead formation based on hydrophobic and hydrophilic principles, soap will orient itself in such a way that the alkyl chain will immerse itself into an oil droplet and the carboxylate will align on the perimeter. This effectively turns an oil droplet into a hydrophilic entity surrounded by hydrophobic alkyl chains.

Recommended topic video on(Colloids)
Some solved examples
Example 1:
Dust storm is an example of a
- (correct) dispersion of solid in gas
- dispersion of gas in solid
- dispersion of solid in solid
- dispersion of solid in liquid
Solution:
In a dust storm, solid particles (dust) are dispersed in the gas phase (air), making it an example of dispersion of solid in gas.
Example 2:
Among the following, the INCORRECT statement about colloids is:
- They can scatter light.
- They are larger than small molecules and have high molar mass.
- (correct) The osmotic pressure of a colloidal solution is of a higher order than the true solution at the same concentration.
- The range of diameters of colloidal particles is between 1 and 1000 nm.
Solution:
The incorrect statement is option (3). The osmotic pressure of a colloidal solution is of small order compared to a true solution at the same concentration.
Example 3:
The colloidal solution of two liquids is called
- Gel
- Aerosol
- (correct) Emulsion
- Liquid Sol
Solution:
A colloidal solution of two liquids is called an Emulsion. Emulsions are stabilized mixtures of immiscible liquids, like oil and water.
Example 4:
A colloidal system consisting of a gas dispersed in a solid is called a /an:
- Gel
- Aerosol
- (correct) Solid sol
- Foam
Solution:
A colloidal system consisting of a gas dispersed in a solid is called a Solid sol.
Conclusion
The paper has stepped into the broad scientific spectrum and involved several colloids under discussion. It has gone about investigation in terms of lyophilic and lyophobic colloids, multimolecular and macromolecular colloids, and colloids relevant to adsorption. It has further interwoven the application of soap in colloidal arrangement within the ambit of wet colloidal science. We have also taken up the practical application of the science of colloids using the example of how soaps help in the process of cleansing. All these types of colloids can throw more light not only on understanding some basic scientific principles but can also lead to developing a richness in the universal presence and critical applications in industrial processes as well as the products we use in our daily lives.
Frequently Asked Questions (FAQs)
It is a mixture in which microscopic dispersed particle size ranges from 1 to 1000 nanometers.
Lyophilic colloids are those colloids that attract the dispersing medium (solvent) and remain stable, but lyophobic colloids are those which repel the dispersing medium, and unsteady and need stabilizing agents to prevent coagulation.
When small molecules combine and give a larger particle which is then suspended into a medium known as a multimolecular colloid.
Associated colloids, for example: micelles - are formed with the aggregation of surfactant molecules at a specific concentration of CMC, such that they trap oils, and dirt to be removed with water.
Soaps clean by forming micelles around the oil and grease particles that disperse them in water to let them be rinsed easily
Also Read
19 Feb'25 04:59 PM
04 Nov'24 10:45 AM
07 Oct'24 12:46 PM
07 Oct'24 12:44 PM
04 Oct'24 06:04 PM
30 Sep'24 02:35 PM
30 Sep'24 02:28 PM
30 Sep'24 11:36 AM