Coordination Number - Overview, Definition, Factor, Examples, FAQs
The total number of atoms, ions, and molecules bonded to the central metal atom in a coordination complex is called the coordination number. Another term used for the coordination number is called ligancy. which means the number of atoms bonded to the central metal atom and it is generally called ligands.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Coordination Number
- Factors That Affect Coordination Number
- How to Find the Coordination Number of Central Atom:
- Coordination Number Examples
- The geometry of Molecules Based on Coordination Number
- Some Solved Examples
In this article, we cover the concept of coordination numbers which is fall under the Coordination Compounds. It is an important topic of class 12 for board exams and also for the Joint Entrance Examination (JEE Main) exam and the National Eligibility Entrance Test (NEET).
What is Coordination Number
Do you know how to define the Coordination number? it is defined as the total number of atoms, ions, or molecules attached to an atom in a specific molecule or crystal and is referred to as the coordination number of that atom. The coordination number of an atom is often referred to as its ligancy.
The ligands are the atoms, ions, or molecules that are attached to the center atom (or molecule/ion). When computing the coordination number of a central atom in a crystal, the legacy of molecules is computed differently.
According to the radius ratio, “The larger the charge, the smaller the ion becomes, limiting the number of groups that can coordinate.”
Factors That Affect Coordination Number
Different types of forces hold the atoms together in these complexes and contribute to the observed coordination numbers. In fluoride complexes, the bonds to the extremely electronegative fluorine atoms are virtually ionic. Therefore increase in coordination number with fluoride ions of 4 to 6 to 7 for B3+, Fe3+, and Zr4+ is primarily due to the cation's increased size. This allows a growing number of fluoride ions to be packed around the center ion.
Also, check-
How to Find the Coordination Number of Central Atom:
The coordination number related to a given atom in polyatomic ions and molecules can be computed by counting the total number of atoms, it is bound. whether it is a single bond or a double/triple bond all are included to calculate coordination number. Using the polyatomic ion [Cr(NH3)2Cl2Br2]– as an example, the coordination number of the core cation (Cr3+) can be calculated by counting the total number of atoms linked to the chromium atom, which is 6. This means the coordination number is 6.
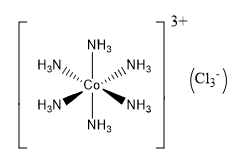
Because the center cobalt atom is connected to six different nitrogen atoms in the example above, the coordination number of the central cobalt atom is six. The bonds between crystals are less obvious in their solid-state forms. In such cases, the coordination number of the center atom reflects the sum arrangement of neutrons around the atom in question. The total number of atoms that surround a specific atom in a crystal is determined by the atom's position in the crystal. In the case of crystals, there are two separate metrics of ligancy- the bulk coordination number and the surface coordination number. Also, students can find related topics below.
Also Check-
- Mohrs Salt
- Quantum Numbers
- Atomic number mass number
- 118 elements, their symbols and their atomic numbers
Coordination Number Examples
A coordination number of a crystalline solid is the number of atoms, ions, or molecules that a central atom/ion has as its nearest neighbors. For example, the coordination numbers of Pt and Fe in the complex ions [PtCl6]2– and [Fe(H2O)6]2+ are 6 and 6, respectively. Pt and Fe are linked to six ligands, Cl and H2O, respectively.
[Cr(NH3)2Cl2Br2]– is another example. Because the total number of atoms/ions/molecules linked to Cr is discovered to be 6, the core atom Cr has coordination number 6. The bidentate ligand, the coordination number Co is 6 in the complex ion [Co(en)3 ]3+. Below students can find coordination numbers with some examples.
[Ag(NH3)2]+, where Ag has a coordination number of 2 and the compound's molecular shape is linear.
[NiCl4]2, where Ni has a coordination number of 4 and the compound's molecular shape is square planar.
[CoCl6]3 is a chemical with the coordination number 6 and an octahedral molecular shape.
The molecular geometry of [ZrF7]3 is a pentagonal bipyramid, with Zr having coordination number 7.
[CoCl5]2 is a chemical with the coordination number 5 and a trigonal bipyramidal molecular shape.
The geometry of Molecules Based on Coordination Number
We can calculate the geometry of a molecule using the coordination number. A list is given below with the corresponding geometry and coordination number.
Coordination number | Molecular Geometry |
2 | Linear |
3 | Trigonal Planar |
3 | T-shaped |
3 | Trigonal Pyramidal |
4 | Tetrahedral |
4 | Square Planar |
5 | Trigonal Bipyramidal |
5 | Square Pyramid |
6 | Octahedral |
7 | Pentagonal Bipyramidal |
7 | Capped Octahedron |
8 | Square Antiprism |
8 | Dodecahedron |
8 | Hexagonal Bipyramidal |
9 and above | Other complex structures |
There are different types of lattices like BCC, FCC, and many more. The abbreviation for BCC is body-centered cubic and The coordination number of the bcc atom is 8. The abbreviated of FCC is Face centered cell and the coordination number is 12. The abbreviation of CCP stands for cubic close-packed and the coordination number is 12. Similarly, hcp stands for hexagonal close-packed cell and the coordination number of hcp is 12. The coordination number of simple cubic seems to have a coordination number of 6 and each unit cell contains one atom.
NCERT Chemistry Notes :
Some Solved Examples
Example. 1
The coordination number of a central metal atom in a complex is determined by
1) (correct)the number of ligands around a metal ion bonded by sigma bonds
2)the number of ligands around a metal ion bonded by pi-bonds
3)the number of ligands around a metal ion bonded by sigma and pi-bonds both
4)the number of only anionic ligands bonded to the metal ion.
Solution
The coordination number is the number of ligands that are bonded directly to metal by coordinate bonds. Thus, the coordination number is the number of ligands around a metal atom/ion bonded by sigma bonds.
Hence, the answer is the option (1).
Example. 2
The coordination number of HCPs is
1)8
2)10
3)6
4) (correct)12
Solution
As we learned in
Coordination number -
The total number of atoms touching a particular atom in the given unit cell is known as the coordination number and those atoms are known as the nearest neighbor.
- wherein
Unit cell Coordination number
- Primitive 6
- BCC 8
- FCC 12
- HCP 12
In HCP packing the coordination number of atoms is 12.6 in the Same plane, 3 in the upper plane, and 3 in the lower plane.
Hence, the answer is the option (4).
Example. 3
The coordination number of copper in Cuprammonium sulfate is
1)2
2)6
3) (correct)4
4)-4
Solution
In Cuprammonium sulfate [Cu(NH3)4]SO4 , there are four ammonia ligands bonded to the central metal ion through coordinate bonds.
Thus, the coordination number of Cu is 4.
Hence, the answer is the option (3).
Also, Refer To
Frequently Asked Questions (FAQs)
There are multiple factors that affect the coordination number like the charge which depends on the electromagnetic configuration of the metal ion, the size of the metal ion, and many more.
A coordination number is a number of legends bounded by central metal. For example, in the given compound [Cr(NH3)2Cl2Br2]−, Cr3+ is a central atom that has six legends bounded with it therefore the coordination number is 6 and the compound is described as a hexacoordinate.
the metal ion plays an important role and the larger the metal ion, the more ligands can fit around it and number of legends is called the coordination number thus higher the size of the metal ion more the coordination number is.
the coordination number depends on the structure of the metal as the larger the size of metal more the legends. as well as it depends on the charge on the metal.
A unit cell is the smallest repeating unit that has full symmetry of the crystal structure and it is defined as a parallelepiped, with the six lattice parameters taken as the lengths of the cell edges (a, b, c) and the angles between them (α, β, γ).
There are some examples of coordination number
[Ag(NH3)2]+, where Ag has a coordination number of 2 and the compound's molecular shape is linear.
[NiCl4]2, where Ni has a coordination number of 4 and the compound's molecular shape is square planar.
[CoCl6]3 is a chemical with the coordination number 6 and an octahedral molecular shape.
The coordination number means a number of atoms surrounded by a central atom. Coordination number of 5 means the central ion is surrounded by 5 molecules or legends.
The coordination number can be determined by examining the molecular structure of a coordination complex. It is the total count of the atoms or ions directly bonded to the central metal.
Common coordination numbers include 2, 4, 6, 8, and occasionally 12. A coordination number of 6 is typical for octahedral complexes, 4 for tetrahedral complexes, and 2 for linear complexes.
Ligands are ions or molecules that donate electron pairs to the central metal ion, forming coordinate bonds. The properties of ligands, such as their size and charge, significantly influence the coordination number, as well as the overall stability and geometry of the complex.
when central metal atom is surrounded by the 12 ligands or atoms and ions . its coordination number is 12 .
Also Read
18 Nov'24 03:03 PM
04 Nov'24 11:17 AM
21 Oct'24 06:24 PM
21 Oct'24 06:14 PM
21 Oct'24 04:19 PM
17 Oct'24 06:11 PM
17 Oct'24 06:00 PM
04 Jul'22 12:16 PM