Defects in Solids
Defects in solids are imperfections in what would otherwise be a regular, periodic array of atoms within a crystalline solid. While ideal crystals have perfect and continuous arrangements of atoms, the truth is that most materials always have a high number of defects. Quite often, these defects account for changes in the properties of materials, like variations in strength, electrical conductivity, or chemical reactivity. Defects can be classified into three broad categories: points, line, and planar defects.
This Story also Contains
- Point Defects, Line Defects
- Metal Excess Defect
- Summary
Point Defects, Line Defects
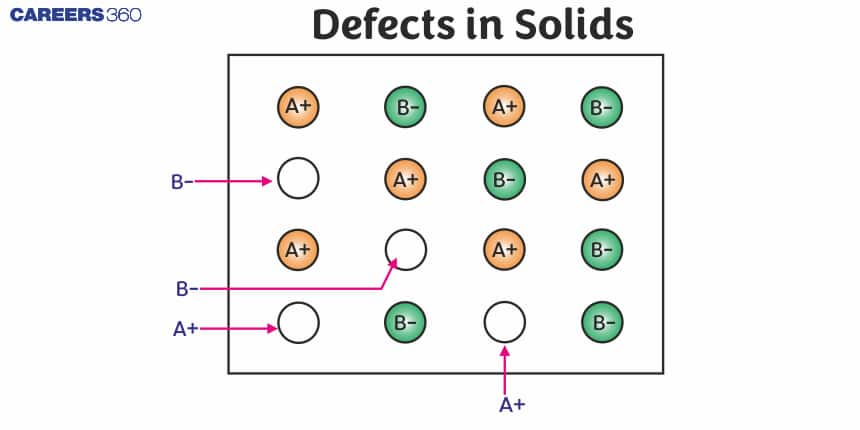
Although crystalline solids have short-range as well as long-range order in the arrangement of their constituent particles, crystals are not perfect. Usually a solid consists of an aggregate of a large number Of small crystals. These small crystals have defects in them. This happens when the crystallisation process occurs at a fast or moderate rate. Single crystals are formed when the process of crystallisation occurs at an extremely slow rate. Even these crystals are not free of defects. The defects are basically irregularities in the arrangement of constituent particles. Broadly speaking, the defects are of two types, namely, point defects and line defects. Point defects are irregularities or deviations from the ideal arrangement around a point or an atom in a crystalline substance, whereas line defects are irregularities or deviations from the ideal arrangement in entire rows of lattice points. These irregularities are called crystal defects. We shall confine our discussion to point defects only.
Types of Point Defects
Stoichiometric Defects
Those compounds in which the number of positive and negative ions are exactly in the ratio indicated by their chemical formula are called stoichiometric compounds example, NaCl. These solids show the following types of defects:
Vacancy Defect: When some of the lattice sites are vacant. the crystal is said to have a vacancy defect.
This results in a decrease in the density of the substance. This defect can also develop when a substance is heated.
Interstitial Defect: When some constituent particles (atoms or molecules) occupy an interstitial site, the crystal is said to have an interstitial defect.
This defect increases the density of the substance. Vacancy and interstitial defects as explained above can be shown by non-ionic solids. Ionic solids must always maintain electrical neutrality. Rather than simple vacancy or interstitial defects, they show these defects as Frenkel and Schottky defects.
Frenkel Defect: This defect is shown by ionic solids. The smaller ion (usually a cation) is dislocated from its normal site to an interstitial site.
It creates a vacancy defect at its Original site and an interstitial defect at its new location. Frenkel defect is also called dislocation defect. It does not change the density of the solid. Frenkel defect is shown by ionic substances in which there is a large difference in the size of ions, for example, ZnS, AgCl, AgBr and Agl due to the small size of Zn2+ and Ag+ ions.
Schottky Defect: It is basically a vacancy defect in ionic solids. In order to maintain electrical neutrality, the number of missing cations and anions is equal.
Like simple vacancy defects, Schottky defect also decreases the density of the substance. The number of such defects in ionic solids is quite significant. For example, in NaCl, there are approximately 106 Schottky pairs per cm3 at room temperature. In 1 cm3 there are about 1022 ions. Thus, there is one Schottky defect per 1016 ions. Schottky defect is shown by ionic substances in which the cation and anion are of almost similar sizes. For example, NaCl, KCl, CsCl and AgBr. It may be noted that AgBr shows both, Frenkel as well as Schottky defects.
Impurity Defects
If molten NaCl containing a small amount of SrCl2 is crystallised, some of the sites of Na+ ions are occupied by Sr2+. Each Sr2+ replaces two Na+ ions. It occupies the site of one ion and the other site remains vacant. The cationic vacancies thus produced are equal in number to that of Sr2+ ions. Another similar example is the solid solution of CdCl2 and AgCl.
Non-Stoichiometric Defects
There are many compounds in which the ratio of positive and negative ions present in the compound differs from the required by the ideal formula of the compound. Such compounds are called Non-stoichiometric compounds. For example, VOx
In these compounds, a balance of positive and negative charges is maintained by having extra electrons or extra positive charges. These defects are of the following types:
Metal Excess Defect
Due to Anionic Vacancies and (F-centres)
A compound may have excess metal ions if a negative ion is absent from its lattice site, leaving a hole which is occupied by an electron to maintain electrical neutrality.
The holes occupied by electrons are called F -centres and are responsible for the colour of the compound.
The excess of sodium in NaCl makes the crystal appear yellow.
Excess of potassium in KCI makes it violet.
Excess of lithium in LiCl makes it pink.
The greater the number of F-centres greater is the intensity of colour. This type of defect is found in a crystal which is likely to possess Schottky defects.
Due to Cationic excess
It may occur if an extra positive ion is present in an interstitial site.
Electrical neutrality is maintained by the presence of an extra electron in the Interstitial site.
These types of defects are exhibited by the crystals which are likely to exhibit Frenkel defects. For example, the yellow colour of ZnS.
Metal Deficiency Defect
The non-stoichiometric compounds may have metal deficiency due to the absence of a metal ion from its lattice site. The charge is balanced by an adjacent ion having a higher positive charge. These types of defects are generally shown by compounds of transition metals. For example, FeS, NiO.
Recommended topic video on (Defects in Solids)
Some Solved Examples
Example 1: The density of crystal suffering from Frenkel defect is:
1)Increases
2)Decreases
3) Remain same
4)All of the above
Solution
In Frenkel defect, cation or anion or both are missing from their correct lattice site and occupy the interstitial space so the mass of crystal is not affected, hence the density of crystal remains the same. In this defect, the density of the crystal remains the same but conductivity increases and the crystal behaves as a p-type semiconductor. This defect is found in the compound having a low coordination number and having a significant size difference between cation and anion.
Ex: transition metal halides, CuCl, CuBr, AgBr, AgI, ZnS, etc.
Hence, the answer is the option (3).
Example 2: As a result of the Schottky defect
1)There is no effect on density
2)Density increases
3)Density decreases
4)All of the above
Solution
In the Schottky defect, cations and anions are missing from their lattice site due to which the mass of the crystal decreases but the volume of the crystal remains the same, this causes a decrease in density. In this defect, the density of the crystal decreases and crystal behaves as a P-type semiconductor, and the conductivity of the crystal increases.
% of the missing unit $=\left(\frac{\mathrm{d}_{\text {theoretical }}-\mathrm{d}_{\text {experimental }}}{\mathrm{d}_{\text {theoretical }}}\right) \times 100$
This defect is common in the compound having a high coordination number and almost equal size of cation and anion.
ex: NaCl, KCl, CsCl, AgBr etc
Hence, the answer is the option (3).
Example 3: F-centres in an ionic crystal are:
1) Lattice sites containing electrons
2)Interstitial sites containing electrons
3)Lattice sites that are vacant
4)Interstitial sites containing cations
Solution
In metal excess defect, some anions are missing from their correct lattice site leaving behind their electron, and the location of the electron is known as F-Centre.
Hence, the answer is the option (1).
Example 4: If NaCl is doped with $10^{-3}$ mol% GaCl3, what is the concentration of the cation vacancies per mole of NaCl?
1)$1.205 \times 10^{15}$
2) $1.2 \times 10^{19}$Correct
3)$12.05 \times 10^{1}$
4)$15.43 \times 10^{17}$
Solution
100 moles of NaCl are doped with $10^{-3}$ moles of $\mathrm{GaCl}_3$. .
Thus, 1 mole of NaCl is doped with $\mathrm{GaCl}_3=10^{-5}$ moles
Now, as we know, as one Ga3+ ion is introduced, three Na+ need to be removed to maintain the electrical neutrality. So as one vacancy is filled by Ga3+ ion, two cation vacancies are formed.
Therefore, the concentration of cation vacancy $=2 \times 10^{-5}$ moles/mole of NaCl
Thus, the number of Cationic vacancies per mole of NaCl
Hence, the concentration of cation vacancies $=1.2 \times 10^{19}$ per mole of NaCl
Hence, the answer is the option (2).
Example 5: Which of the following compounds is likely to show both Frenkel and Schottky defects in its crystalline form?
1) AgBr
2)CsCl
3)KBr
4)ZnS
Solution
Frenkel Defect: This defect is shown by ionic solids. The smaller ion (usually a cation) is dislocated from its normal site to an interstitial site.

It creates a vacancy defect at its Original site and an interstitial defect at its new location. Frenkel defect is also called dislocation defect. It does not change the density of the solid. Frenkel defect is shown by ionic substances in which there is a large difference in the size of ions, for example, ZnS, AgCl, AgBr and Agl due to the small size of Zn2+ and Ag+ ions.
Schottky Defect: It is basically a vacancy defect in ionic solids. In order to maintain electrical neutrality, the number of missing cations and anions is equal.

Like a simple vacancy defect, the Schottky defect also decreases the density of the substance. The number of such defects in ionic solids is quite significant. For example, in NaCl, there are approximately 106 Schottky pairs per cm3 at room temperature. In 1 cm3 there are about 1022 ions. Thus, there is one Schottky defect per 1016 ions. The Schottky defect is shown by ionic substances in which the cation and anion are of almost similar sizes. For example, NaCl, KCl, CsCl and AgBr. It may be noted that AgBr shows both, Frenkel as well as Schottky defects.
Only AgBr can exhibit both Schottky and Frenkel defects.
Therefore, Option(1) is correct.
Summary
Defects in solids refer to the imperfections of the otherwise regular arrangement of atoms in the crystal. This would include point defects dealing with missing atoms or impurities, such as vacancies, interstitials, or substitutional atoms, line defects like dislocations, and planar defects like grain boundaries. They can fundamentally alter the material's properties, including its mechanical strength and electrical conductivity.