Dehydration of Alcohols (Dehydrogenation) - Mechanism, Examples, FAQs
After reading this article, the reader should be able to answer the following questions- What is formed when a primary alcohol undergoes catalytic dehydrogenation, write the mechanism of dehydration of ethanol to ethene, give examples of dehydrating agent, give mechanism of acid catalyzed dehydration of ethanol to ethene, How can you convert alkene to alcohol, give hydration of alkene mechanism, primary alcohol undergo what reaction to form alkenes, what do you mean by dehydration, what is dehydrogenation, what is dehydrogenation of alcohol, what is dehydrogenation meaning in hindi.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
Note: Dehydrate meaning in Hindi is निर्जलीकरण, dehydration meaning in hindi is निर्जलीकरण
Dehydration chemistry is highly useful in industrial processes. The literal meaning of the word ‘dehydration’ is to get rid of water and in this case, molecules of alcohol are getting rid of water. Dehydration of alcohol is an example of an elimination reaction where OH and H bonded to two adjacent carbon atoms get eliminated to form a double bond between the same carbon atoms.
Elimination reactions are of two types. E1 type of elimination reaction is considered for tertiary alcohols and E2 type of elimination reaction is considered for Primary alcohols. The dehydration of alcohol gives alkene. There are a few conditions required for dehydrogenation of ethanol reaction like high temperature and presence of Bronsted acids.
- Dehydration chemistry is highly dependent on the dehydration reagents.
- Dehydration of alcohol with Al2O3 mechanism is carried out in the presence of solid Al2O3
- Examples of dehydrating agents are concentrated Sulfuric acid, ZnCl2, and H3PO4, and H2SO4.
- A few changes in the conditions can lead to the formation of ether from alcohol in place of alkene. The mechanism of dehydration of alcohol to form ether follows a SN2 reaction at lower temperatures.
- There are about 6-7 methods of dehydration in whole chemistry for different compounds but Dehydration of alcohol is an example of substitution or elimination reaction.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Following is an example of acidic dehydration of alcohol.
- Dehydration of butan-1-ol is given as follow-
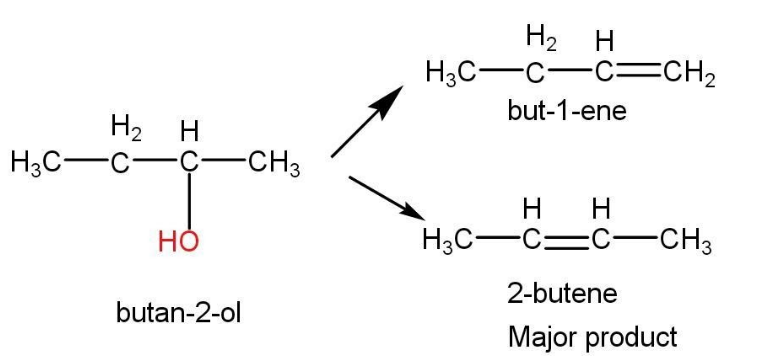
Dehydration of glycerol-

Alcohol to alkene-
Shown below is a general reaction for dehydration of alcohols to alkene (dehydration of ethanol)
General reaction for the conversion of alcohol to alkene-

- Since dehydration of alcohol is an example of an elimination reaction. Therefore, High temperatures are essential to favor this reaction.
- Bronsted acids are essential because they are proton donors. We will see how proton donors play a crucial role in the alcohol dehydration mechanism. Bronsted acids are also known as dehydrating agents. H2SO4 and H3PO4are some of the examples of dehydrating agents that are used to carry out the dehydration of alcohols.
- Acid-catalyzed dehydration of alcohol mechanism depends on the type of alcohol i.e., whether alcohol is primary, secondary or, tertiary will decide the path of mechanism.
Mechanism of Tertiary alcohol and secondary alcohol-
Dehydration mechanism for tertiary/Secondary alcohols:
3⁰ alcohols are relatively easily dehydrated Under mild conditions which means these will dehydrate even in 20 % aqueous acid solution and a temperature of 85⁰C whereas these conditions change drastically for primary alcohols.
Following is the step-by-step mechanism for acidic Dehydration of alcohol to alkene-
Let us consider the alcohol dehydration reaction of tert-butyl alcohol:
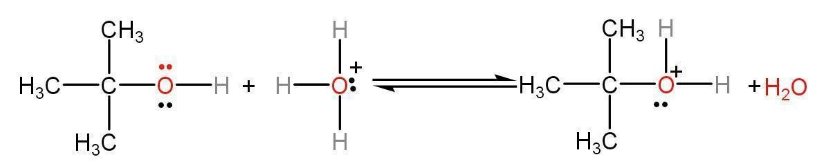
Step 1: In the first step of dehydration of tertiary alcohol oxygen gets protonated from the acid catalyst. We can see how a proton donor is required to initiate the dehydration of alcohol to alkene. Hence it is acid-catalyzed dehydration of ethanol to ethene reaction. Hydronium ions of the acid get transferred to the oxygen atom of the alcohol which has an unshared pair of electrons. A positive charge develops on the oxygen atom and all the oxygen bonds become weaker. H2O will act as a leaving group.
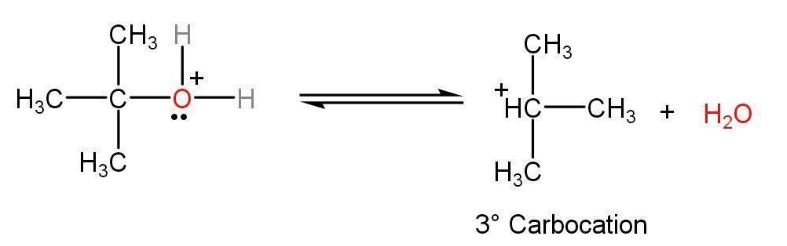
Step 2: H2O leaves as leaving group and carbocation is generated. This carbocation is the intermediate step in this step. This carbocation is reactive because it has only six electrons in its valence shell.
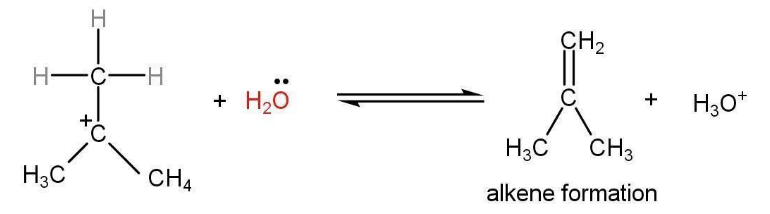
Step 3: Hydrogen atoms are removed from the β-Carbon of the carbocation and an alkene is formed with a hydronium ion.
Dehydration mechanism of Primary alcohols:
Dehydration of ethanol to ethene takes place in drastic conditions. Alcohol dehydration reactions require higher temperatures and concentrated acid solutions. Dehydration of primary alcohol such as ethyl alcohol follows an E2 elimination reaction path because the intermediate formed in this mechanism is secondary carbocation. The mechanism of acid-catalyzed dehydration of ethanol gives ethene.
Dehydration of ethanol reaction proceeds by the formation of carbocation but the conditions required are different since primary carbocation is not more stable than the tertiary and secondary. Dehydration of ethanol follows the mechanism of acid-catalyzed dehydration of ethanol to ethene.
Following is the step-by-step mechanism for acidic dehydration of ethanol-
Step 1: Alcohol mechanism of action is different because it is not a good leaving group. The oxygen of primary alcohol shares its electron with hydronium ions and a protonated alcohol is formed. We know that the -OH group is not a good leaving group but with the protonation of the -OH group H2O+ can leave as a leaving group.
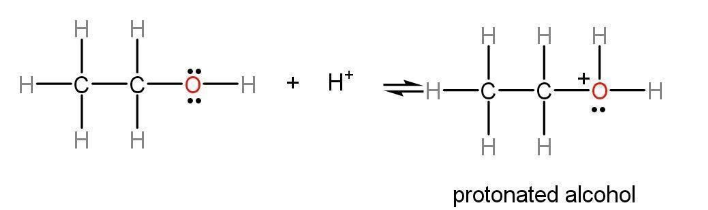
Step 2: In step 2 the H2O+ group leaves and a carbocation is formed. The formation of the carbocation in dehydration of ethanol is the rate-determining step of its mechanism.
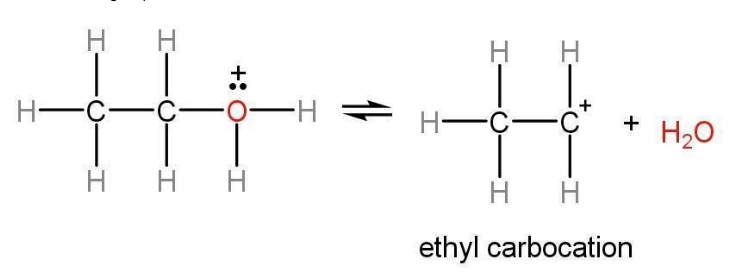
Step 3: Carbocation is unstable as it only has six electrons in its valence shell. Therefore, it releases H+ and an alkene i.e., ethane, rate-determining is formed from ethyl alcohol. Dehydration of ethanol mechanism is the general mechanism for the dehydration of alcohol.
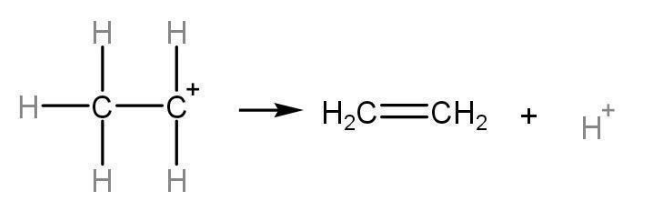
Fig- Dehydration of ethanol
Above is the mechanism of acid-catalyzed dehydration of alcohol -
Dehydration of alcohol in case of secondary alcohol-
- A good example of secondary alcohol is cyclic alcohol such as Cyclohexanol.
- Secondary alcohols get dehydrated in milder conditions such as 85% Acid solution and 170⁰C temperature.
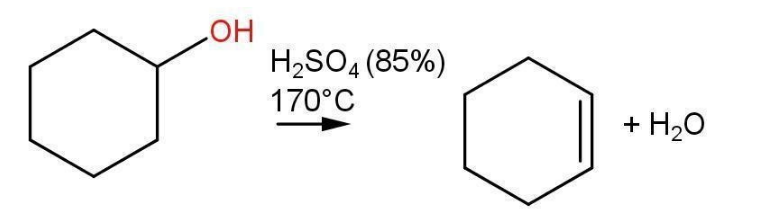
Fig- Dehydration of cyclohexanol
Order of dehydration of alcohol-
- Order of dehydration of alcohol will depend upon its structure i.e., tertiary, secondary atom reactions, or primary. The slowest rate-determining step in both the mechanisms in which a carbocation is formed.
- The type of carbocation formed will determine the ease with which the reaction will follow. E1 reaction is followed by tertiary and secondary alcohols in which the intermediate form is tertiary and secondary carbocation respectively. A tertiary and secondary carbocation is stable as compared to primary hence, the free energy of activation is lower.
- However, the E2 reaction is followed by primary alcohols. The intermediate form is a primary carbocation which is the least stable carbocation among all the others and the free energy of activation is higher. Dehydration of ethyl alcohol will be the slowest among all.
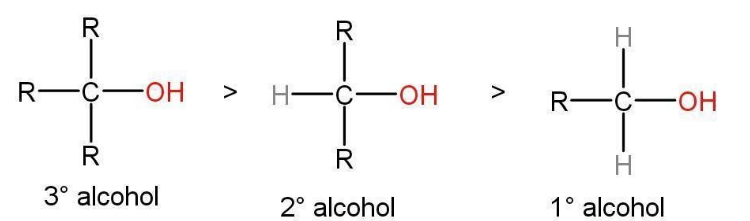
Fig- The rate of dehydration of alcohol follows the order mentioned above.
Alkene to Alcohol (acid-catalyzed hydration of alkene)-
Reversal of the above reaction will give us alkene. Acid-catalyzed hydration of alkene is an example for an addition reaction. In industries, Reaction to convert alkene to alcohol is very useful to produce low- molecular-weight alcohols.
Hydration of alkene Mechanism is as follows –
Acid is required for the initiation of this reaction and is called the acid-catalyzed mechanism of hydration of alkenes.
- Markovnikov’s Rule- To convert alkene to alcohol this rule is followed for the addition of water to an alkene. It says that the positive entity of the reagent will add to the carbon atom with the most number of hydrogen and the negative entity of the reagent will add to the carbon with a lesser number of hydrogens.
Following is the mechanism of acid-catalyzed hydration of alkene-
Step 1- The dehydration mechanism starts with the formation of the carbocation is the first step and the rate-determining step. A more stable carbocation is formed in this step.
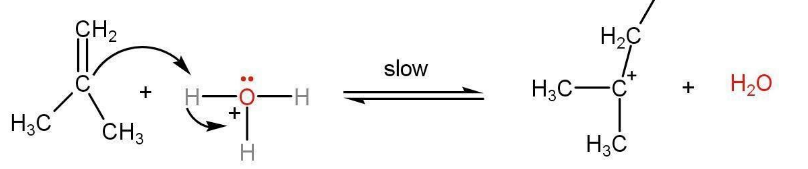
Step 2- Attack of a water molecule on carbocation to share the lone pair of the oxygen atom. Protonated alcohol is formed.
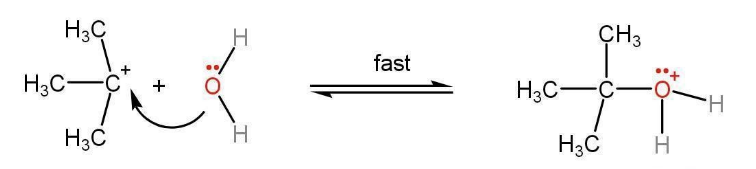
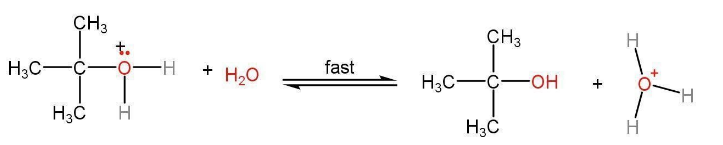
Step 3- Water present in the solution takes away the proton to form alcohol.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11 Alcohols, Phenols and Ethers
- NCERT notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
What is Dehydrogenation of alcohol?
Dehydrogenation process in which hydrogen is removed from the substrate. Usually, these reactions are useful for organic molecules such as alkanes, formaldehyde. Dehydrogenations meaning lies in its name i.e., removal of hydrogen from a molecule or addition of oxygen. Since Organic molecules containing carbon are highly stable due to catenation property a large amount of energy is required to remove a wanted molecule from the substrate. Hence, the Dehydrogenation reaction is an endothermic process.
Following are a few types of Dehydrogenation reactions-
- Dehydrogenation of alkanes-To converts saturated fats to unsaturated fats.
- Ethylbenzene to styrene.
- Paraffins and olefins to pentene and iso-pentene.
- Methanol to formaldehyde (addition of oxygen)
Catalytic dehydrogenation of alcohol can be carried out in the presence of catalysts. These catalysts can be either homogeneous or heterogeneous.
Examples-
- The catalytic dehydrogenation reaction of alcohol gives aldehydes.
- Alcohol metabolism in the body is regulated by Alcohol dehydrogenase (ADH)
Alcohol dehydrogenase reaction is given as follows-
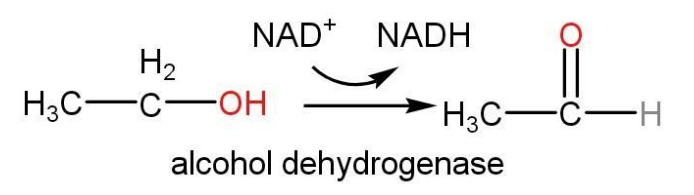
Dehydrogenation reaction of alcohol to aldehyde requires the surface of catalysts such as copper. This reaction follows β-elimination with the atom reactions, hydrogen and -OH group.
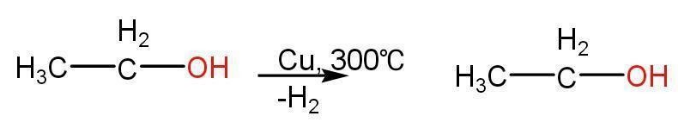
- Dehydrogenation reaction of alkanes- Dehydrogenation of alkanes gives olefins. Alkanes are stable and hence not reactive. Therefore, converting alkanes to alkenes is important to obtain essential precursors to form alcohols, aldehyde polymers, etc.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Catalytic dehydrogenation of 1⁰ alcohol gives an aldehyde. H2 is removed from the substrate. Catalytic dehydrogenation of primary alcohol can be initiated on Ag catalysts in presence of oxygen. Many times catalysts such as Pt, Pd are also used in absence of oxygen.
Primary alcohols undergo an electrophilic elimination reaction to form alkenes. Tertiary and secondary alcohols follow the E1 path and Primary alcohol follows the E2 path.
Protonated alcohol is formed in the E1 pathway and the formation of carbocation takes place.
Dehydration meaning is to remove the molecule of water from a substrate. Alcohols undergo a dehydration reaction.
Dehydration of alkenes takes place in such a way that more substituted alkene is formed. When there are two more carbons from where hydrogen can be eliminated then in that case that hydrogen is removed which yields a more substituted alkene. The intermediate formed in the elimination reaction (E1) is a carbocation. Sometimes to form the most stable carbocation rearrangement of carbocation takes place. This carbocation will decide the alkene formed in the product.
Tertiary alcohol is most easily dehydrated due to the presence of the -OH group in the tertiary position. In this case, protonated alcohol is formed at a tertiary position to form a tertiary carbocation.
Prop-2-ane, Ethene, But-1-ene
Acid-catalyzed hydration of alkenes follows an electrophilic addition reaction. In the first step, there is the formation of a carbocation. The most reactive alkene will be the one that forms the most stable carbocation. Prop-2-ane will form a tertiary carbocation. Hence, propene will be the most reactive alkene.
Also Read
30 Sep'24 11:38 AM
30 Sep'24 08:59 AM
21 Jul'22 03:44 PM
16 Jul'22 05:44 PM
16 Jul'22 05:33 PM
16 Jul'22 05:08 PM
16 Jul'22 05:01 PM

