Endothermic Reaction - Definition, Examples, Differences, FAQs
Define Endothermic Reaction.
To understand this concept, we need to keep in mind the following things:
1) Bond breaking requires energy
2) Bond formation releases energy
Let us consider an example, the reaction of vinegar and baking soda results in the formation of sodium acetate, water and carbon-di-oxide. Before forming the products, atoms of the molecules need to rearrange themselves. Atoms are attracted to one other; therefore, it requires energy to pull them apart. The rearrangement of the atoms of molecules gives us the product. Different amounts of energy are required to form the molecules in the reactant and the product side. Comparing its energy difference insights us into whether a chemical reaction is absorbing or releasing energy.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Define Endothermic Reaction.
- What is an Endothermic Reaction?
- How to Identify Exothermic and Endothermic Reactions?
- Difference Between Exothermic Reaction and Endothermic Reaction
- Endothermic Reaction Examples Equations.
- Energy Diagram
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is an Endothermic Reaction?
Endothermic Reaction Definition: Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The reactions lower the temperature of their surrounding area, thereby creating a cooling effect.
How to Identify Exothermic and Endothermic Reactions?
We can't observe bond breaking and formation, but we can do the following to distinguish between exothermic and endothermic reactions:
1) Temperature change can be monitored using a thermometer. Endothermic reactions happen with a decrease in temperature as it takes energy from the system. When the system temperature is increased, we can note it down as an exothermic reaction.
2) Enthalpy change:
The summation of energy used in reactant bond breaking and energy used in product bond making is known as enthalpy. It is a measure of internal energy. The energy released in breaking a bond in the reactant is always positive and negative during bond making on the product side.
If enthalpy change (∆H) is negative, an exothermic reaction occurs because more energy is released while forming the product than breaking it.
If enthalpy change (∆H) is positive, more energy is consumed to break the bond on the reactant side, and less energy is released when products are formed; then endothermic reaction takes place.
Related Topics Link |
Difference Between Exothermic Reaction and Endothermic Reaction
1) Endothermic process examples reaction absorbs energy from the surrounding
Exothermic reaction liberates energy to the surrounding.
2) Temperature decreases in case of endothermic reaction while temperature increases in an exothermic reaction
3) Potential energy of the product side is higher in an endothermic reaction. And, the potential energy of the reactant side is greater than the product side in case of an exothermic reaction.
4) Surrounding entropy decreases in an endothermic reaction, and entropy increases in an exothermic reaction.
Endothermic Reaction Examples Equations.
1) Photosynthesis is responsible for the existence of living beings. This is an endothermic process because green plants absorb energy from the sunlight (surrounding) to yield glucose and oxygen.
2) Melting of ice is an endothermic process. Water molecules are packed in a rigid form in the ice. It absorbs energy from the surroundings as heat. Water molecules move faster as the temperature increases and ice melts down.
3) Baking bread and cooking eggs are also endothermic processes; energy is absorbed from the oven or pan to cook it.
4) Sublimation of dry ice: sublimation occurs at a temperature and pressure below the critical point. The phase change requires energy to convert from a solid to a gas phase.
5) Instant ice pack to treat injuries.
SOME OTHER ENDOTHERMIC REACTION EQUATIONS:
1) N2(g) + O2(g) +heat 2NO(g)
2) MgCO3+ heat MgO+ CO2
3) NH4NO3 + heat NH4+ + NO3-
Read more :
- NCERT notes Class 11 Chemistry Chapter 6 Thermodynamics
- NCERT solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 6 Thermodynamics
Energy Diagram
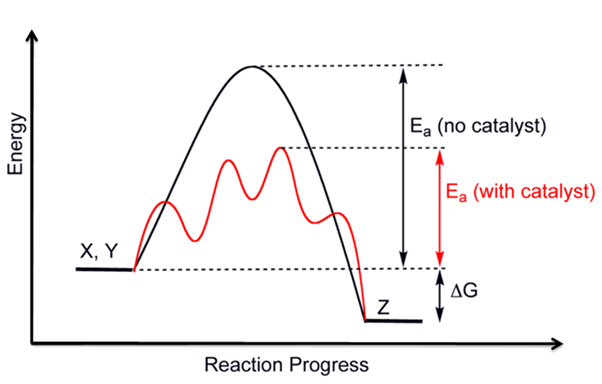
NCERT Chemistry Notes :
In chemistry, there is an energy profile showing relative potential energy with time. It is based on the fact that energy changes in bond breaking and bond formation. While bond formation, bonds can be first partially broken and partially formed. This is called the transition state, which is more significant in energy than both reactant and product energy. It is an unstable state, and energy is required to cross the barrier to reach the product formation side. The minimum energy needed to cross this barrier is called activation energy.
This minimum energy is the energy difference between reactant and transition state. The relation between the activation energy and enthalpy change for any chemical reaction can be determined from the energy profile. In endothermic reactions, the overall ∆H is positive because energy is provided to cross the energy barrier, and the product is less stable. In an exothermic reaction, the potential energy of the product is less compared to the reactant. Hence, the product is more stable in case of an exothermic process.
Also check-
Frequently Asked Questions (FAQs)
Endothermic process because energy is provided to perform the reaction that eventually breaks the bond and rearranges to give us the product.
The reaction must be endothermic because it takes energy from the surroundings. Here, it takes it from the water also thereby decreasing its temperature.
2AgBr 2Ag + Br2
This is an endothermic reaction example.
2HBr H2 + Br2
BOND | BOND ENERGY(kJ/mol) |
H-Br | 336 |
H-H | 436 |
Br-Br | 193 |
Calculate the enthalpy change and comment on the type of reaction.
Answer: enthalpy change is given by the formula-
∆Htotal = ∆ Hproduct - ∆ Hreactant
= - (436+193) kJ/mol - (2x-336) kJ/mol
= (-629 + 732) kJ/mol
= +103 kJ/mol
It is an endothermic reaction.
X (g) + heat X+(g) + e-
Here, ionization is happening. The energy required to remove an electron from its valence orbital in the gaseous phase is called ionization energy.
Since there is an attractive force between electron and nucleus in an atom, we need to break that force.
Therefore, the formation of cation from an atom in the gas phase is an example for endothermic reaction.
Also Read
19 Feb'25 12:52 PM
19 Feb'25 10:40 AM
19 Feb'25 10:36 AM
19 Feb'25 10:35 AM
19 Feb'25 10:16 AM
18 Feb'25 11:45 PM
18 Feb'25 11:43 PM
18 Feb'25 11:40 PM
18 Feb'25 11:38 PM
18 Feb'25 11:36 PM