Ester - Definition, Formula, Structure with FAQs
What is an Ester?
A chemical compound derived from an organic or an inorganic acid where the substitution of the hydroxyl group takes place by an alkoxy group is termed as an ester. Esters are formed as a result of the reaction between an oxoacid and a compound containing a hydroxyl group like phenol or alcohol. Esters tend to produce alcohol and salt (organic or inorganic) on reacting with water. Esters are one of the most encountered functional groups in chemistry.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- What is an Ester?
- Ester Meaning-
- What is the formula of an ester?
- Naming esters-
- Ester IUPAC name-
- Ester structure –
- Ethyl Ester-
The carbon atom is attached to three different atoms-
A single bond between two carbon atoms
A double bond between carbon and oxygen
A single bond between carbon and oxygen where oxygen is further bonded to the carbon atom by a single bond.
Ester Meaning-
Any of a category of compounds produced as a result of the reaction between an acid and an alcohol with the elimination of a water molecule is called an ester.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is the formula of an ester?
The universal formulation of an ester can be depicted as –
R-COO-R', where R can represent a hydrogen atom, an alkyl group or an aryl group and R' can represent an alkyl or aryl group but not a hydrogen atom.
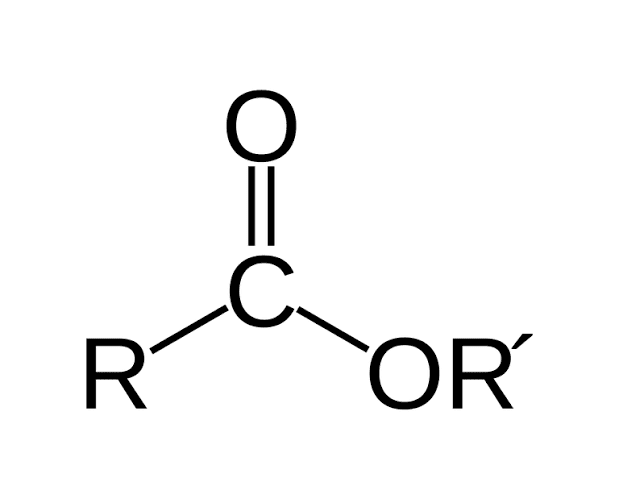
This is the ester chemical formula.
Naming esters-
The naming of esters is based completely upon the alcohol and the acid from which it is derived. Here we'll learn how esters are named.
What are the IUPAC and common names of the given ester molecule?
Ans: Esters molecules are composed of two molecules coming together especially carboxylic acids and alcohol. If you look carefully at the structure above, you'll notice that the first three carbons are derived from the carboxylic acid part of the molecule. We notice that it has C=O and is single bonded to oxygen. Normally in a carboxylic group, you'll have, -OH and nothing else.
So that's the carboxylic acid part and either side of the carbonyl group is derived from alcohol. Imagine we'd had -OH attached to the three-carbon chain and no carbonyl group. So that becomes alcohol. With that being said we begin naming this by looking at the alcohol part of the molecule. We have CH2-CH2-CH3 which is three carbon long and can be named propane. But since this is an alkyl group, we drop the -ane part and name it as propyl. This is the first part of the name in this molecule.
The second part of the name is the ionic term carboxylic acid so the three-carbon long chain with carbonyl group is propanoic acid. But the molecule is an ester, so we need to drop the -oic from acid and call it propanoate and that's the ionic name for carboxylic acid. Putting these two names together we get – 'propyl propanoate'. The common name for propyl propanoate is propionate. The above-mentioned procedure is to be followed for naming ester compounds.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Ester IUPAC name-
We are aware of the fact that the esters formed are a combination of an acid and alcohol where the hydrogen atom of the acid part is substituted by an alkyl group of alcohol with the elimination of water molecules. Therefore, -COO is the functional group of esters or the ester group. The ester name is formed of two words, the first one derived from an alkyl group that replaces the hydrogen atom and the second one being derived from the acid part. Hence, the common term for an ester is alkyl alkanoate.
Following steps are to be followed for IUPAC naming of an ester-
Encounter the ester functional group -COO
Identification of the alkyl chain of carboxylic acid.
The naming of the acid.
Rewriting the suffix -oic acid to -oate.
Identification of the alkyl group that had replaced the hydrogen atom from acid.
Naming this alkyl group.
Rearrangement of the words by putting firstly the name of an alkyl group from alcohol followed by the name of the alkyl group of carboxylic acid.
Leaving space between these two words.
| Related topics link, |
Ester structure –
Let us take the example of ethyl ester – ethyl propanoate. Ethyl propanoate is formed as a result of the reaction between propanoic acid and ethyl alcohol or ethanol by the esterification process. The general ester formula for ethyl ester is
Steps to be followed for drawing ethyl ester structure:
Identification of the alkyl group and the term alkanoic acid i.e propanoic acid.
Drawing the structure of ethanoic acid. The carbon atom of the carbon-oxygen double bond is the initial atom in the chain.
Removal of a hydrogen atom bonded to oxygen from propanoic acid.
Using the name of the alkyl group.
Joining the carbon atoms by a dash representing a covalent bond between the carbon atoms. It should be initiated from the oxygen having lost the hydrogen atom.
Completing the structure by fulfilling the valency required.
So the required structure of ethyl propanoate is-
Ethyl Ester-
Ethyl esters on hydrolysis yield ethyl alcohol. Omega 3 ethyl esters are obtained from fish oil which is eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). They are available in two forms – triglycerides and ethyl esters. While within the ethyl ester form, the glycerol backbone is missing. Therefore, the fatty acids will find an available triglyceride backbone or take one from an existing molecule.
If the latter occurs, the molecule missing the backbone will search for another backbone, and so on, creating a consequence. The free fatty acids are haunted by the enterocytes (gut epithelium) and must be reconverted to triglycerides to be transported within the blood. Fats are stored and transported within the body in triglyceride form.
This is supported by our understanding of human physiology: when ethyl esters are consumed, they're processed within the liver, where the ethanol is drawn off, and therefore the body must then rebuild the resulting free fatty acids back to a triglyceride. The ethyl esters that get digested produce free fatty acids plus ethanol.
This is often certainly a less efficient absorption process compared with the direct intake of a natural form of triglyceride because the ethyl esters form must be reconverted within the body back to a triglycerides form. The delay in triglycerides re-synthesis suggests that transport to the blood is more efficient in natural triglycerides fish oils as compared to ethyl esters. Furthermore, this delay of triglycerides re-synthesis in ethyl esters fish oils causes a release of ethyl alcohol and should subsequently produce oxidative stress by releasing free radicals additionally to releasing the ethanol.
Research shows that after ingestion of an omega-3 carboxylic acid molecule in triglyceride form, the fatty acids are cut from the glycerol backbone, the backbone and fatty acids are absorbed via the gut epithelial cells and immediately reattached to make the natural triglyceride.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The process followed to produce an ester as the main product by reaction between an acid and an alcohol.
The reaction between the carboxylic acid and an ester is carried out in presence of sulphuric acid or hydrochloric acid
Esters are used in the preparation of synthetic flavours, perfumes, Some of the volatile esters are used as solvents
In coatings, paints and varnishes
Esters usually smell sweet. The presence of weak intermolecular forces between the esters makes it volatile. Hence, this volatile nature makes esters smell.
Ethyl butanoate or ethyl butyrate has a structural formula of C6H12O2.
Ethyl ethanoate, methyl propanoate
In 1g of substance, the amount (mg) of potassium hydroxide needed to react with esters is the ester value.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM
Articles
Questions related to
Correct Answer: Acrolein
Solution : The correct option is Acrolein.
Acrolein is used in the production of acrylic acid and its esters, which are essential in the manufacture of various polymers and materials. Additionally, acrolein has applications as a biocide and herbicide. Acrolein is highly toxic.