Friedel Crafts Acylation Alkylation - Meaning, Definition, Process, Limitation, FAQs
What are Friedel Crafts Reactions?
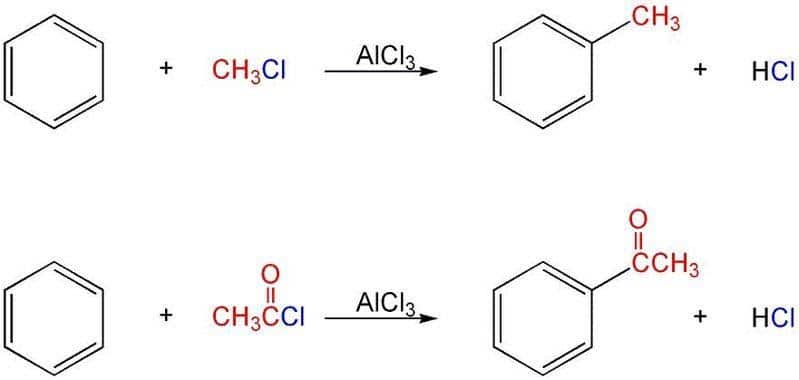
The Friedel Crafts reaction is an electrophilic substitution reaction that produces a hydrocarbon or ketone by replacing the hydrogen atom of an aromatic molecule with an alkyl or acyl group. In the presence of an acid catalyst, such as AlCl3, BF3, ZnCl2, FeCl3, and so on, the aromatic molecule is alkylated or acylated.The catalyst produces the attacking particle, an alkyl or acyl cation.C. Friedel and J. Crafts discovered this reaction in 1877-1878, and used it to attach substituents to an aromatic ring.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- What are Friedel Crafts Reactions?
- Friedel Crafts Alkylation Mechanism
- Friedel Crafts Alkylation Mechanism
- Limitations of Friedel - Crafts Alkylation Reaction
- Friedel Crafts Acylation Mechanism
- Limitations of Friedel Crafts Acylation
- Acylation of Phenol
- Acylation of Anisole
It should be noted that the hydrogen atom that is initially connected to the aromatic ring is replaced with an electrophile in both alkylation and acylation procedures. Aluminium trichloride is the most widely utilised catalyst because it acts as a Lewis acid by combining with the halogen to produce an efficient electrophile.
Because of the positive charge on the carbon atom, alkyl and acetonic groups act as electrophiles in this process. These groups target the haloarene's electron-rich ortho and para locations, resulting in the major product being the para isomer and the minor product being the ortho isomer.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Friedel Crafts Alkylation Mechanism
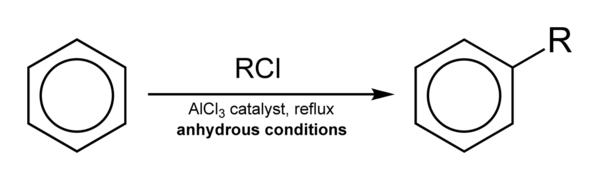
Friedel-Crafts Alkylation is defined as the substitution of an alkyl group for an aromatic proton. With the help of a carbocation, an electrophilic attack on the aromatic ring is carried out. The Friedel-Crafts alkylation reaction uses alkyl halides as reactants to generate alkyl benzenes.
In this reaction, a Lewis acid catalyst such as FeCl3 or AlCl3 is used to facilitate the elimination of the halide and so generate a carbocation. Before proceeding with the alkylation step, the resultant carbocation undergoes a rearrangement.
Friedel Crafts Alkylation Mechanism
A three-step process controls the Friedel-Crafts alkylation reaction.
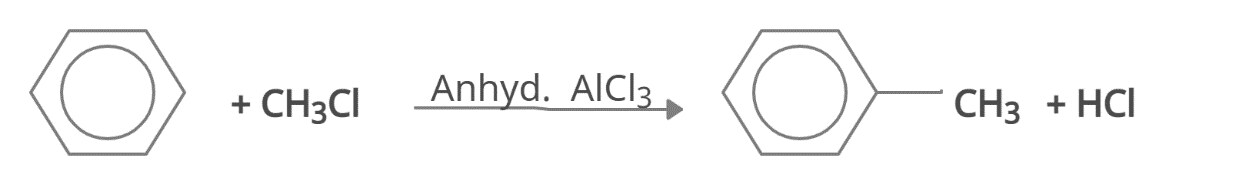
The formation of a methyl carbocation from methylbromide is the first step in the mechanism of Friedel-Crafts Alkylation Reaction. The carbocation then combines with the benzene electron system to generate a nonaromatic carbocation that loses a proton, restoring the system's aromaticity.
Step 1:
The alkyl halide reacts with the Lewis acid catalyst (AlCl3), resulting in the production of an electrophilic carbocation is the first step in the mechanism of Friedel-Crafts Alkylation.
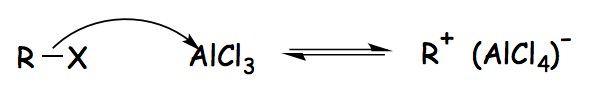
Friedel-Crafts Alkylation Mechanism- Step 1
Also read :
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Step 2:
After attacking the aromatic ring, the carbocation forms a cyclohexadienyl cation as an intermediate. Due to the breakdown of the carbon-carbon double bond, the aromaticity of arene is temporarily lost.
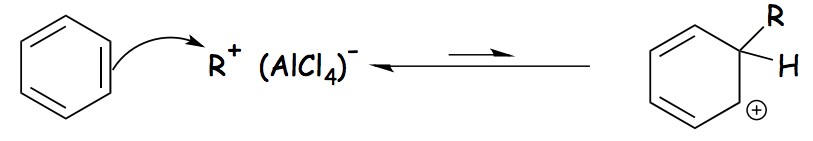
Friedel-Crafts Alkylation Mechanism- Step 2
Step 3:
The deprotonation of the intermediate causes the carbon-carbon double bond to regenerate, restoring the compound's aromaticity. The AlCl3 catalyst is regenerated when this proton reacts with hydrochloric acid to create hydrochloric acid.
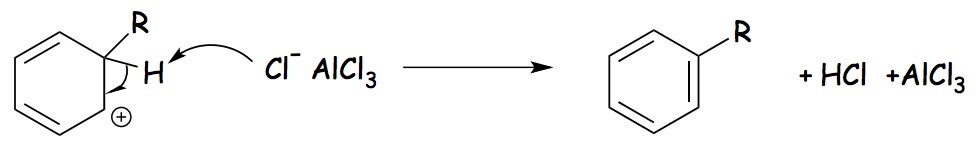
Friedel-Crafts Alkylation Mechanism- Step 3
Limitations of Friedel - Crafts Alkylation Reaction
This reaction cannot utilise the carbocation produced by aryl and vinyl halides since they are exceedingly unstable.
A deactivating group (such as an NH2) on the aromatic ring might cause the catalyst to deactivate due to complex formation.
To avoid polyalkylation, an excess of the aromatic component must be utilised in these processes (addition of more than one alkyl group to the aromatic compound).
The Friedel-Crafts alkylation reaction does not include aromatic compounds that are less reactive than mono-halo benzenes.
It is important to note that this reaction, like any other involving carbocation, is susceptible to carbocation rearrangements.
Related Topics link, |
What is Friedel Crafts Acylation Mechanism?
An acyl group is added to an aromatic ring in the Friedel Crafts acylation mechanism procedure. A Lewis acid catalyst, such as AlCl3, and an acid chloride (R-(C=O)-Cl) are commonly utilised. The aromatic ring is converted to a ketone via a Friedel-Crafts acylation mechanism process. Under these conditions, the reaction between benzene and an acyl chloride is shown below.
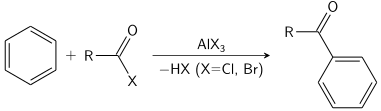
Friedel Crafts Acylation Mechanism
Friedel Crafts acylation mechanism are carried out in a four-step process.
Step 1
The Lewis acid catalyst (AlCl3) and the acyl halide undergo a reaction. The acyl halide loses one of its halide ions, generating an acylium ion that is stabilised by resonance.
The remainder of the mechanism is the same as for benzene alkylation. There are no rearrangements because the acylium ion is resonance stabilised.
Step 2
After that, the acylium ion (RCO+) attacks the aromatic ring electrophilically. As a complex forms, the ring's aromaticity is temporarily lost.
Step 3
The intermediate complex has now been deprotonated, restoring the ring's aromaticity. HCl is formed when this proton joins a chloride ion (from the complexed Lewis acid). The AlCl3 catalyst will be regenerated.
Step 4
In the final step of the mechanism of Friedel Crafts Acylation the carbonyl oxygen is now attacked by the regenerated catalyst. As a result, by adding water to the products created in step 3, the ketone product must be liberated.
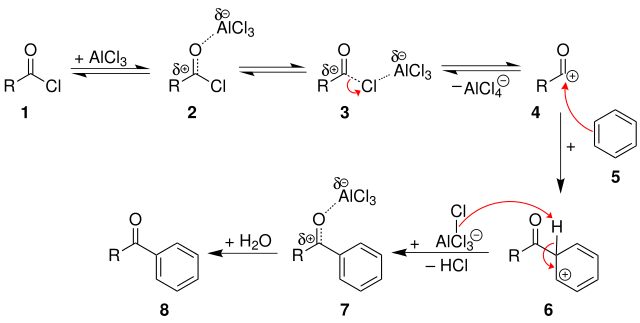
Friedel-Crafts Acylation Mechanism
NCERT Chemistry Notes:
Limitations of Friedel Crafts Acylation
We cannot use aryl amines in this process because they create highly unreactive complexes with Lewis acid catalysts.
In this reaction, the aromatic compound, which is less reactive than mono halo benzene, cannot be employed.
Ketones are the only products of acylation processes.
When amines or alcohols are employed, acylations can occur on the nitrogen or oxygen atoms.
Acylation of Phenol
Friedel-Crafts alkylation can occur with phenols. It is preferable to utilise reagents that can produce the electrophile without using Lewis acids. Friedel-Crafts acylation on phenols necessitates more difficult circumstances, such as a high temperature. Because the phenol forms a combination with AlCl3, its activity is reduced. Phenols are bivalent nucleophiles, meaning they can react on the aromatic ring to produce an aryl ketone via C-acylation, a Friedel-Crafts reaction, or on the phenolic oxygen to produce an ester via O-acylation, an esterification.
Acylation of Anisole
In the presence of anhydrous aluminium chloride as a catalyst, anisole reacts with acetyl chloride to produce 2-methoxy acetophenone and 4-methoxy acetophenone. The ortho – para group of directors is the Methoxy group. The reaction will be continued by Friedel-Crafts alkylation, in which the Lewis acid catalyst separates the methyl chloride ion from the chloride ion, resulting in a volatile, electrophilic methanium ion. The electrophilic ion is then struck by chlorobenzene electrons from the Π mechanism of the benzene ring.
Also check-
Frequently Asked Questions (FAQs)
Friedel-Crafts acylation uses a Lewis acid catalyst and an acyl chloride to add benzene to an acyl ring and offers a few benefits over alkylation. The ketones produced can be converted to alkyl groups by Clemmensen reduction.
According to the theory, Friedel-Crafts alkylation was considered to be reversible. Alkyl groups in the presence of protons or other Lewis acids are removed in a retro-Friedel-Crafts process or Friedel-Crafts dealkylation. The true result of the reaction is 1, 3, 5-triethylbenzene, which has all alkyl groups as a meta substituent.
The acylium ion is the electrophile in Friedel Crafts acylation reaction. The acylium ion is resonance stabilised and has a positive charge on the carbon. As an electrophile, this acylium ion reacts with the arene to produce the monoacylated product (aryl ketone).
Alkylation is the process of substituting an alkyl group - in this case, a benzene ring – with something else. A group like methyl or ethyl, for example, is substituted for a hydrogen ring. Benzene is treated with chloroalkane in the presence of aluminium chloride as a catalyst.
The Friedel-Crafts acylation reaction is one of the most frequent aromatic chemistry procedures utilised in the synthesis of aryl ketones. When stoichiometric amounts of Lewis acid are used, a complex forms at the end of the reaction between the aryl ketone produced and the Lewis acid.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM