Gattermann Reaction - Mechanism, Examples, Application, FAQs
The Gattermann reaction, ( which is also known to be the Gattermann formylation or the Gattermann salicylaldehyde synthesis reaction ) is a type of chemical reaction through which an aromatic compounds will be formylated with the help of a mixture of hydrogen cyanide i.e.HCN and hydrogen chloride i.e. HCL in the presence of a Lewis acid catalyst for example AlCl3. This reaction is named after a German chemist Ludwig Gattermann and this reaction is similar to the Friedel–Crafts reaction.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Q- Gattermann reaction
- Applications of Gattermann Formylation Reaction
- Q- Gattermann reaction class 12?
- Points to know:-
This reaction can be easily understand by changing the HCN/AlCl3 combination with zinc cyanide. Despite that it is also very toxic, Zn(CN)2 is a solid, and making it safer to work in this reaction than gaseous HCN. Then Zn (CN)2 will be able to reacts with HCl for the synthesis of a very important HCN reactant and Zn (Cl)2 then will serves as the Lewis-acid catalyst in in-situ condition. An example of the Zn (CN)2 used method is in the formation of mesitaldehyde from mesitylene.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Q- Gattermann reaction
This reaction is Named after:
A German chemist, known as Ludwig Gattermann
The gattermann reaction can be discussed as a method in the process of formylation of aromatic ring compounds. Another names of this reaction are Gattermann salicylaldehyde synthesis and gattermann formylation. The gattermann reaction mechanism is very similar to that of Friedel-Crafts reaction.
Another use of Gattermann reaction is the preparation of halobenzene from benzenediazonium salt by treating with Cu/HX.
Q- Explain Gattermann reaction mechanism?
Step 1: The Formation of Formimino Chloride when HCN reacts with HCl for the synthesis of formimino chloride and leads to the formation of Formimino Chloride.
Step 2: Next step will be the Formation of Electrophile: Formimino chloride when reacts with a lewis acid catalyst like AlCl₃ then it will form Formimino cation.
Step 3: There will be Attack of Electrophile on Benzene Ring: The formimino Cation then reacts with the benzene ring to become Benzaldimine by the process of Attack of Electrophile on Benzene Ring
Step 4: There will be Hydrolysis of Benzaldimine: Hydrolysis of benzaldimine occurs which results to the formation of benzaldehyde.
Q- Gattermann synthesis?
It is the process that is used in the synthesis of aromatic ring compounds for example aromatic halides or aromatic aldehydes. This process is very similar to that of the Friedel-Crafts reaction. This process is named after a German Chemist Ludwig Gattermann. This process is also known to be Gattermann Formylation. In this reaction for the synthesis of aromatic halide diazonium salt will reacts with copper powder in presence of respective halogen acid. This reaction is generally a type of substitution reaction.
Applications of Gattermann Formylation Reaction
This reaction is used for the synthesis of chlorobenzene and bromobenzene.
Thia reaction is used for the synthesis of benzaldehydes.
Products of Gattermann formylation reaction like benzaldehydes and haloarenes etc. Are widely used in various fields like pharmaceuticals, agricultural, medicinal etc.
This reaction is used for the synthesis of aromatic halides and aromatic aldehydes.
Formation of Aromatic Aldehyde through Gattermann Reaction
Gattermann Formylation Reaction Mechanism
Mechanism of Gattermann Reaction have to be explained for the synthesis of aromatic aldehydes. Reaction takes place through the given four steps
Step 1. Synthesis of Formimino Chloride
Hydrogen cyanide when reacts with hydrogen chloride then it forms formimino chloride as a product.
Step 2. Synthesis of Electrophile
Formimino chloride when reacts with lewis acid catalyst (like AlCl3) will forms formimino cation.
Step 3. Attacking of Electrophile to the Benzene Ring
Formimino cation (electrophile) will attacks on the benzene rings and leads to the formation of benzylamine.
Step 4. Hydrolysis or addition of water to Benzylamine
Hydrolysis of benzylamine will be taken place in this step. Which then results in the synthesis of benzaldehyde.
Related Topics Link, |
How is Diazonium Salt will be Formed?
Aromatic amine when reacts with nitrous acid and mineral acid then it will forms diazonium salt and also produces water as a by product. This reaction is well known as Diazotization Reaction.
Gattermann reaction of diazonium salt is shown below.
ArNH2 + HNO2 + HX ? RN2+X- + H2O
Aromatic amine nitrous acid mineral acid Diazoniumsalt water
Q- Gattermann reaction class 12?
Gattermann Reaction → In this reaction halogen group is added to the benzene ring when treated with solution of benzene diazonium salt along with halogen acid in presence of copper powder
Gattermann Reaction example
C6H5−N2 + Cl−Cu + HCl (X=Cl,Br) --> C6H5−Cl + N2
It is the process that is used in the synthesis of aromatic ring compounds for example aromatic halides or aromatic aldehydes. This process is very similar to that of the Friedel-Crafts reaction. This process is named after a German Chemist Ludwig Gattermann. This process is also known to be Gattermann Formylation. In this reaction for the synthesis of aromatic halide diazonium salt will reacts with copper powder in presence of respective halogen acid. This reaction is generally a type of substitution reaction.
Q- Gattermann aldehyde synthesis or Gattermann aldehyde reaction?
This reaction involves carbon compounds and the derivatives of carbon due to this property this reaction is known as organic reactions. Gatterman aldehyde synthesis is a type of an organic reaction that is used in the formation of aromatic aldehydes.
Carbon or the derivatives of carbon will be considered as organic compounds and the reactions including organic compounds will be known as organic reactions. Gatterman aldehyde synthesis is a type of an organic reaction and as we can easily observe in its name that this reaction is used to form aldehydes. This process is mostly used in case of aromatic compounds due to the fact that stable products are available only for aromatic compounds. The aromatic compounds will be converted into aldehydes with the help of hydrogen cyanide, hydrogen chloride and a Lewis acid like aluminium chloride.
The reaction will be represented as follows:
Step1.
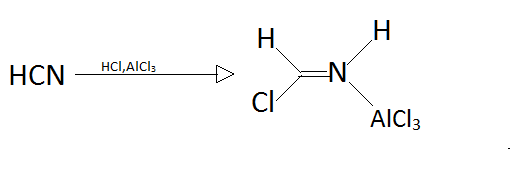
Step2.
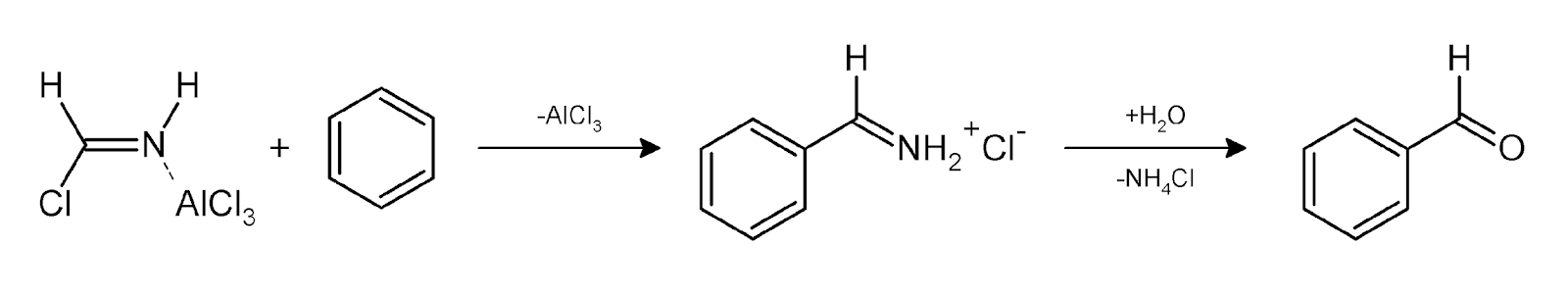
In step one as we can observe that hydrogen cyanide will reacts with hydrochloric acid and aluminium chloride to form a new better nucleophile that can attack on aromatic compound like benzene.
In step two the reagents attacks on benzene will forms a product releasing aluminium chloride and on further reacting with water it again releases ammonium chloride and then we will get our final product benzaldehyde.
So this is the mechanism of Gatterman aldehyde synthesis reaction.
Gatterman aldehyde synthesis reaction is very similar to that of friedel craft reaction. As we know when an alkyl group will be added to a benzene ring through electrophilic substitution reaction, then this process is called as friedel craft alkylation and the same mechanism is happening in gattermann aldehyde synthesis and only the difference is that the group attached is different, in this process we are adding an aldehyde group to the benzene ring.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
- NCERT notes Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Points to know:-
A reaction that is similar to Gatterman aldehyde synthesis also exists in organic chemistry and that reaction is known as Gatterman Koch synthesis. The functional group added in both the reactions are same and the only difference is that their reagents are not same. Carbon monoxide is used as reagent in Gatterman Koch synthesis.
Q- What is gattermann reaction ?
In this reaction benzene diazonium salt is reacted with halogen acid in presence of copper catalyst to form chloro benzene or bromo benzene.
C6H5−N2 + X−Cu + HX (X=Cl,Br) --> C6H5−X + N2
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Also Read
13 Dec'24 09:35 AM
12 Dec'24 04:35 PM
13 Nov'24 05:00 PM
18 Oct'24 11:58 AM
30 Sep'24 08:52 AM
17 Jun'22 05:48 PM
17 Jun'22 04:12 PM
17 Jun'22 04:06 PM
16 Jun'22 06:51 PM
