Isomerism in Coordination Complexes
Isomerism in coordination complexes is a captivating aspect of coordination Chemistry that reveals the intricate ways in which ligands can arrange themselves around a central metal ion. This phenomenon leads to compounds that, while sharing the same molecular formula, exhibit distinct structural and spatial configurations. In Coordination chemistry, isomerism can be broadly classified into two main categories: structural isomerism and stereoisomers.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Geometrical Isomerism
- Optical Isomerism
- Structural Isomerism - 1
- Structural Isomerism - 2
- Stability of Complexes and Real-Life Applications
- Some Solved Examples
- Summary
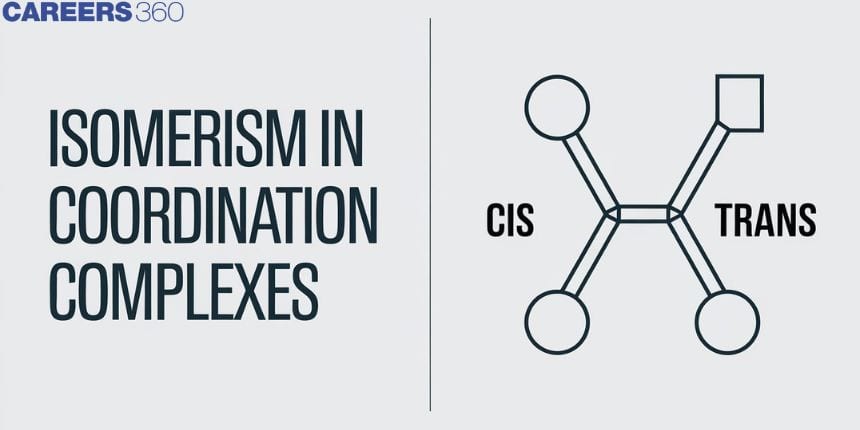
Geometrical Isomerism
This isomerism is due to ligands occupying different positions around the central metal atom or ion. The ligands occupy positions either adjacent or opposite to one another. This type of isomerism is also known as cis-trans isomerism. When two same ligands are at a right angle(90o), the form is cis- form, and when they are present diagonally at 180o to each other, the form is termed as trans- from. Geometrical isomerism is very common in coordination number 4 and 6 complexes.

Another type of geometrical isomerism occurs in octahedral coordination entities of the type M a_3 b_3like mathrm{Co}left(mathrm{NH}_3right)_3left(mathrm{NO}_2right)_3 If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer. When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer.

Optical Isomerism
Optical isomers are mirror images that cannot be superimposed on one another. These are called as enantiomers. The molecules or ions that cannot be superimposed are called chiral. The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left). Optical isomerism is common in octahedral complexes involving bidentate ligands.

Structural Isomerism - 1
Structural isomerism in coordination complexes arises when compounds have the same molecular formula but differ in the connectivity of their atoms. This can occur through various mechanisms, including:
Linkage Isomerism
Linkage isomerism arises in a coordination compound containing ambidentate ligands. A simple example is provided by complexes containing the thiocyanate ligand, NCS–, which may bind through the nitrogen to give M–NCS or through sulfur to give M–SCN. Jørgensen discovered such behavior in the complex [Co(NH3)5(NO2)]Cl2, which is obtained as the red form, in which the nitrite ligand is bound through oxygen (–ONO), and as the yellow form, in which the nitrite ligand is bound through nitrogen (–NO2).
Coordination Isomerism
This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex. An example is provided by [Co(NH3)6][Cr(CN)6], in which the NH3 ligands are bound to Co3+ and the CN– ligands to Cr3+. In its coordination isomer [Cr(NH3)6][Co(CN)6], the NH3 ligands are bound to Cr3+ and the CN– ligands to Co3+.
Structural Isomerism - 2
The second aspect of structural isomerism is geometric isomerism, which refers to the spatial arrangement of ligands around the metal center. This is particularly relevant in octahedral and square planar complexes.
- Geometric Isomerism: In octahedral complexes, for example, the complex [Co(NH3)4Cl2]+ can exist as cis and trans isomers. In the cis isomer, the two chloride ions are adjacent to each other, while in the trans isomer, they are opposite each other.
- Facial and Meridional Isomerism: For complexes with the formula [MA3B3][MA3B3], where AA and BB are different ligands, two types of isomers can occur: facial (fac) and meridional (mer). In fac isomers, three identical ligands occupy one triangular face of the octahedron, while in mer isomers, they are arranged in a plane bisecting the octahedron.
These geometric variations can lead to different physical properties, such as melting points and solubility, which are crucial in applications ranging from catalysis to drug design.
Ionisation Isomerism
This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion. An example is provided by the ionization isomers [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4.
Solvate Isomerism
This form of isomerism is known as ‘hydrate isomerism’ in cases where water is involved as a solvent. This is similar to ionization isomerism. Solvate isomers differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice. An example is provided by the aqua complex [Cr(H2O)6]Cl3 (violet) and its solvate isomer [Cr(H2O)5Cl]Cl2.H2O (grey-green).
Stability of Complexes and Real-Life Applications
The stability of coordination complexes is influenced by their isomeric forms. Factors such as ligand type, charge, and steric play a significant role in determining the stability of a complex. For instance, chelating ligands, which can form multiple bonds to a metal ion, typically enhance stability due to the chelate effect. In real-life applications, isomerism in coordination complexes is pivotal. In pharmaceuticals, the efficacy and safety of drugs can depend on the specific isomer used. For example, cisplatin, a well-known anticancer drug, has distinct therapeutic effects compared to its trans isomer, which is significantly less effective. Additionally, in catalysis, the isomeric form of a catalyst can influence reaction pathways and product distributions. The ability to selectively produce one isomer over another can lead to more efficient and environmentally friendly processes. Case studies in materials science also highlight the importance of isomerism. For example, the development of new materials with specific optical or electronic properties often relies on the precise arrangement of ligands in coordination complexes.
The stability of a complex in solution refers to the degree of association between the two species involved in the state of equilibrium. The magnitude of the equilibrium constant (stability or formation) for the association, quantitatively expresses the stability. Thus, if we have a reaction of the type:
M+n L⇌MLn
The larger the stability constant, the higher the proportion of MLn that exists in the solution. Free metal ions rarely exist in the solution so M will usually be surrounded by solvent molecules which will compete with the ligand molecules, L, and be successively replaced by them. For simplicity, we generally ignore these solvent molecules and write the respective stability constants as follows:
$\begin{aligned} & \mathrm{M}+\mathrm{L} \rightleftharpoons \mathrm{ML} \quad K_1=[\mathrm{ML}] /[\mathrm{M}][\mathrm{L}] \\ & \mathrm{ML}+\mathrm{L} \rightleftharpoons \mathrm{ML}_2 \quad K_2=\left[\mathrm{ML}_2\right] /[\mathrm{ML}][\mathrm{L}] \\ & \mathrm{ML}_2+\mathrm{L} \rightleftharpoons \mathrm{ML}_3 \quad K_3=\left[\mathrm{ML}_3\right] /\left[\mathrm{ML}_2\right][\mathrm{L}] \\ & \mathrm{ML}_{(\mathrm{n}-1)}+\mathrm{L} \rightleftharpoons \mathrm{ML}_{\mathrm{n}} \quad K_4=\left[\mathrm{ML}_{\mathrm{n}}\right] /\left[\mathrm{ML}_{(\mathrm{n}-1)}\right][\mathrm{L}]\end{aligned}$
where K1, K2, ....Kn, etc., are stepwise stability constants. The overall stability constant (β) of the formation of species MLn from M and L can be given as:
$\mathrm{M}+\mathrm{nL} \rightleftharpoons \mathrm{ML}_{\mathrm{n}} \quad \beta=\left[\mathrm{ML}_{\mathrm{n}}\right] /[\mathrm{M}][\mathrm{L}]^{\mathrm{n}}$
The stepwise and overall stability constant are therefore related as follows:
$\beta_{\mathrm{n}}=K_1 \times K_2 \times K_3 \times K_4 \ldots \ldots K_{\mathrm{n}}$
Recommended topic video on (Isomerism in Coordination Complexes )
Some Solved Examples
Example 1: Which of the following compounds show optical activity?
1) Ethane-1,2-diamine
2) [Co(NH₃)₆]³⁺
3) [Co(en)₃]³⁺
4) [Co(H₂O)₆]³⁺
Solution:
Ethane-1,2-diamine (en) is optically active because it does not contain any plane of symmetry or a center of symmetry. Therefore, the correct answer is option (1).
Example 2: Which of the following compounds have the possibility of enantiomerism?
1) [Co(NH₃)₆]³⁺
2) [Co(en)₃]³⁺
3) [Co(H₂O)₆]³⁺
4) All of these
Solution:
Among the given options, only [Co(en)₃]³⁺ is optically active as it does not have symmetry elements. Thus, the correct answer is option (2).
Example 3: The number of geometrical isomers for [Pt(NH₃)₂Cl₂] is:
1) 2
2) 1
3) 3
4) 4
Solution:
The square planar complex [Pt(NH₃)₂Cl₂] can exist in two geometrical forms: cis and trans. Therefore, the correct answer is option (1).
Example 4: Which of the following does not have an optical isomer?
1) [Co(NH₃)₃Cl₃]
2) [Co(en)₃]Cl₃
3) [Co(en)₂Cl₂]Cl
4) [Co(en)(NH₃)₂Cl₂]Cl
Solution:
The complex [Co(NH₃)₃Cl₃] has a plane of symmetry in both of its geometrical isomers, making it optically inactive. Thus, the correct answer is option (1).
Summary
In summary, isomerism in coordination complexes is a multifaceted topic that encompasses various forms of structural and geometric isomerism. Structural isomerism includes ionization and linkage isomerism, while geometric isomerism focuses on the spatial arrangement of ligands around the central metal ion. The stability of these complexes is influenced by their isomeric forms, which is crucial for understanding their behavior in real-life applications.The implications of isomerism are particularly significant in fields such as pharmaceuticals, where the specific isomer used can determine a drug's efficacy and safety. For example, the anticancer drug cisplatin is effective in its cis form but not in its trans form. In catalysis, the isomeric form of a catalyst can affect reaction pathways, thereby influencing product distributions and overall efficiency.
Also Read
18 Nov'24 03:03 PM
04 Nov'24 11:17 AM
21 Oct'24 06:24 PM
21 Oct'24 06:14 PM
21 Oct'24 04:19 PM
17 Oct'24 06:11 PM
17 Oct'24 06:00 PM
04 Jul'22 12:16 PM