Latent Heat of Fusion - Definition, Explanation, Formula, Examples, FAQs
Introduction - Latent Heat of Fusion
In 1761 Joseph Black deduced the application of heat to ice. He found that there is no rise in the temperature of ice/water, but on the other hand, there is an increase in the amount of water in that mixture. Black also observed that there is no increase in heat to boiling water but an increase in the amount of steam can be observed, so he observed that the heat which is supplied, is combined with the particles of ice and to boiling water and become latent in nature, this marks the beginning of thermodynamics related to the theory of latent heat. In this section first, we discuss latent heat than the latent heat of fusion. Without changing the temperature but a change in its physical state by the release or absorption of energy is so called Latent heat.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Introduction - Latent Heat of Fusion
- What is Heat of Fusion?
- Define Latent Heat of Fusion.
- Explain Latent Heat of Fusion.
- Define Specific Heat of Fusion.
- Latent Heat of Fusion Examples:
- Define Enthalpy of Fusion:
- Heat of Solidification:
- Latent heat of Sublimation Definition:
Also read -
What is Heat of Fusion?
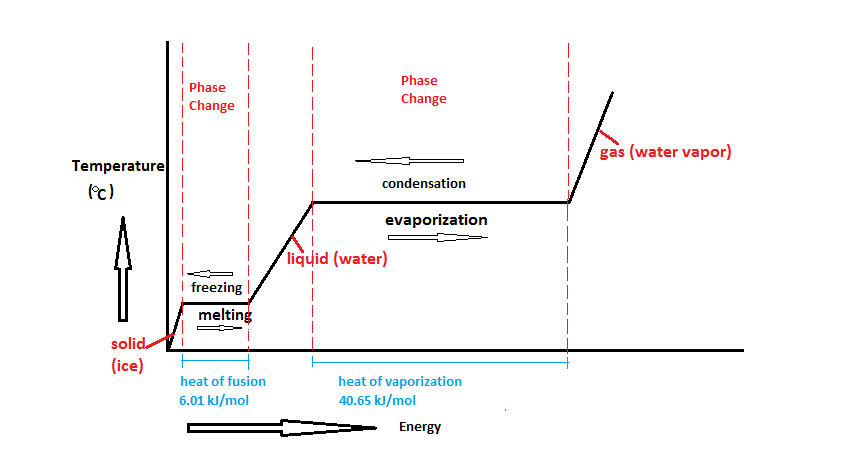
The latent heat of fusion is basically change in enthalpy observed in the melting of solid and freezing of liquid.
Define Latent Heat of Fusion.
Defining the latent heat in scientific terms we can write the definition as the amount of heat released or absorbed per mole in the units of joule or calories while undergoing a change in its state is called the latent heat of fusion. Materialistically we can define it as to overcome the forces that hold up the atoms or molecules together in any materials such required work is termed as Latent heat of fusion. For converting the physical state of any solid material, an amount of energy must be supplied to trigger the change in enthalpy of fusion (the latent heat of fusion). The enthalpy of fusion or latent heat of fusion occurs in constant environmental conditions.
Explain Latent Heat of Fusion.
An increase in the amount of substance which is by volume of the substance is the latent heat of fusion. A phase transition that occurs at this point is due to temperature and is termed the melting point of a substance. Change in phase can occur due to temperature rise or fall, falling of temperature may result in solidification of a substance which is termed as the heat of solidification. The heat of solidification is the opposite of the latent heat of fusion. In the whole process of latent heat of fusion, the atmospheric pressure is considered to be 1.
Generating the vibrations inside the object is the effect of heat. The heat is considered as raised entropy of particles or kinetic energy. Heat always rises but in the case of temperature, high and low can be possible. Latent heat of fusion is different for every state of matter that is solid, liquid and gas. It should be kept in mind the fact that temperature remains constant, additionally, we can add or subtract the heat (latent heat) in an object during the phase change.
Formula for Latent Heat of Fusion will be as Follows: Latent heat of fusion is considered to be written as Q, m will be the mass and latent heat can be denoted as therefore the formula is:
∆Q=mL
Which is the general required form of the equation for the latent heat of fusion. Here the symbol delta Q is used for heat energy in joules, The formula is given accordingly as, when m kg of mass of any solid changes to liquid at its constant temperature and which is at its melting point, then the heat absorbed by the solid is given by the above mentioned equation. In some cases, it can be seen that the temperature varies where we have to take two temperatures i.e. lower temperature and higher temperature for concluding the formula of latent heat of fusion. So heat gain or release, in that case, will be as follows:
∆Q=mC×∆t
∆Q=mC×(t2-t1)
Here the two temperatures are higher and lower temperatures, Total of the latent heat of fusion energies gain or release can be represented:
Q=mL+m×C×∆t
The term latent heat of fusion can also be used as specific latent heat of fusion.
Define Specific Heat of Fusion.
Specific latent heat of fusion can be defined as the amount of heat that is being required to change the state of substance by unit mass and it can be represented as
L= Q/m
Which can be written as Q=mL
Latent heat of fusion of water or specific latent heat of water is the same as 334 joules per gram.
Also read :
- NCERT notes Class 11 Chemistry Chapter 6 Thermodynamics
- NCERT solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 6 Thermodynamics
Latent Heat of Fusion Examples:
If in case 64500 calories of heat are extracted from the given mass of 100g of steam at a temperature of 100℃.Given that the latent heat of fusion of steam and ice are 80 calories per gram and 540 calories per gram respectively. Find the amount of water converted into ice.
The temperature will definitely fall from 100℃ to 10℃, when the full steam is converted into water.
A part of the water is also converting into water at 0℃.
The heat gain by water at 100℃ for conversion of steam at 100℃
C=m×L
C=100×540cal
=54000cal.
Now heat gain by water at 100℃ for conversion into the water at 0℃.
C=100×1×(100-0)
=10000cal
Therefore, the extracted heat =54000cal+ 10000cal=64000cal.
The remaining heat from the given heat will be =64500cal-640000=500cal.
Now, as according to the question the amount of water that is converted to ice is
m=Q/Lice
m=500/80
m=6.25g.
Hence, we concluded the result that the amount of water that is converted into ice is 6.25g.
Related Topics Link, |
Define Enthalpy of Fusion:
As we discussed earlier in the section that fusion generally means melting, changing of solid to liquid requires the energy or heat at its melting point that is called Heat of fusion. But the opposite of this is different as removal or taken away the energy or heat from something results in freezing. The heat of fusion is simply measured in energy terms as in joules per gram or calories per gram. It is to be noted that the heat of fusion is not at all the same for all the substances, but having constant value for each individual substance. The heat of fusion can also be represented in terms of enthalpy of fusion both are the same.
Formula for concluding heat of fusion can be written as follows:
∆Hf=q/m
Here, ∆Hf sows the heat of fusion, q is heat joule and m is mass in kg.
Enthalpy of fusion of ice when heat energy is supplied to one kilogram of water for the conversion of water to one kilogram of ice with no change in the temperature of the environment which means at zero degree Celsius is 333.55kilojoules.
Heat of Solidification:
Earlier we were discussing latent heat of fusion, but the opposite of latent heat of fusion is the heat of solidification. In order to change the phase from liquid to solid, the amount of energy required is called the heat of solidification. It is important to note that the value of latent heat of fusion and heat of solidification is the same in magnitude but differs in sign.
The temperature point at which the freezing is started is termed the heat of solidification. Example of solidification, the energy required to convert the water into ice is the same as the energy required to convert ice into the water but of different signs. The latent heat of fusion is commonly positive except in the case of Helium which means the heat of solidification generally comes out to be negative.
Solidification is always an exothermic process, the heat released during the process.
NCERT Chemistry Notes:
Latent heat of Sublimation Definition:
This is simply defined as the amount of heat or enthalpy that must be added to a solid of one mole at a constant pressure to convert directly into gas with no change in the phase of liquid. All the forces, ions, or molecules are broken down and it comes in the state of the gas.
The unit of latent heat of sublimation can be given in joules per mole or kilogram of substance and represented as ∆Hs.
Also check-
Frequently Asked Questions (FAQs)
Hardening of lava into solid rock.
Snow formation
Melted candle into the wax
This is the process where we get the power for the planets like the Sun and the stars. The reaction occurs between two atoms of hydrogen when combined together to form an atom called helium. Here in the process of fusion some of the mass of hydrogen is converted into energy. The process requires higher temperature as when they are heated to the maximum they get ionized and form plasma. The sun and the stars can do this by gravity. It is an inexhaustible source of energy.
The examples of latent heat are as follows:
Heat of vaporization
Heat of freezing
The heat of solidification is considered as the opposite of it and also the sign conventions are used differently in each but are the same in magnitude.
The change in enthalpy observed in the melting of solid and freezing of liquid.
Also Read
19 Feb'25 12:52 PM
19 Feb'25 10:40 AM
19 Feb'25 10:36 AM
19 Feb'25 10:35 AM
19 Feb'25 10:16 AM
18 Feb'25 11:45 PM
18 Feb'25 11:43 PM
18 Feb'25 11:40 PM
18 Feb'25 11:38 PM
18 Feb'25 11:36 PM