Lattice Enthalpy, Hydration Enthalpy And Enthalpy Of Solution
Lattice enthalpy is the change in energy that takes place when one mole of any ionic compound is formed from its gaseous ions under standard conditions, usually taken to be at 298 K and under a pressure of 1 bar. Because the formation of strong ionic lattice bonds occurs, it is always negative and exothermic. High lattice enthalpy means that the ionic compound is pretty stable because lots of energy will be released when a lattice forms. Thus, for example, when solid sodium chloride NaCl is formed from the gaseous ions of sodium, Na⁺, and chloride, Cl⁻, much energy is released because the ions are united by very strong electrostatic forces.
This Story also Contains
- Lattice Enthalpy
- Heat of Hydration
- Heat of Solution
- Solubility Of an Ionic Compound In Water
- Some Solved Examples
- Summary
Lattice Enthalpy
The lattice enthalpy of an ionic compound is the enthalpy change that occurs when one mole of an ionic compound is formed from its ions in a gaseous state.
$\mathrm{Na}^{+}(\mathrm{g})+\mathrm{Cl}^{-}(\mathrm{g}) \longrightarrow \mathrm{NaCl}(\mathrm{s}), \Delta \mathrm{H}=-788 \mathrm{kJmole}^{-1}$
Heat of Hydration
The enthalpy change during hydration of one mole of any gaseous ion is called heat of hydration.
$\begin{aligned} & \mathrm{Na}^{+}(\mathrm{g}) \longrightarrow \mathrm{Na}^{+}(\mathrm{aq}), \Delta \mathrm{H}=\Delta \mathrm{H}_{\mathrm{hyd}_{\mathrm{Na}^2}+} \\ & \mathrm{Cl}^{-}(\mathrm{g}) \longrightarrow \mathrm{Cl}^{-}(\mathrm{aq}), \Delta \mathrm{H}=\Delta \mathrm{H}_{\mathrm{hyd}_{\mathrm{Cl}^{-}}} \\ & \end{aligned}$
Heat of Solution
It is a change in enthalpy when one mole of a solid solute is dissolved more than the solvent.
$\mathrm{NaCl}(\mathrm{s}) \longrightarrow \mathrm{Na}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq}), \Delta \mathrm{H}=\Delta \mathrm{H}_{\mathrm{sol}_{\mathrm{NaCl}}}$
Solubility Of an Ionic Compound In Water
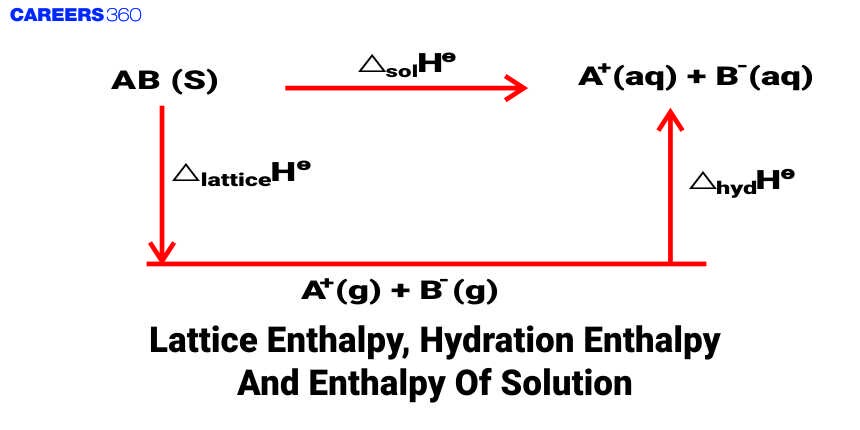
When an ionic compound dissolves in a solvent, the ions leave their ordered positions on the crystal lattice. These are now more free in solution. But solvation of these ions (hydration in case the solvent is water) also occurs at the same time. The enthalpy of solution of any ionic solid, in water is, therefore, determined by the selective values of the lattice enthalpy and enthalpy of hydration of ions. This will be more clear with the help of the diagram given below:

Thus, the enthalpy of solution, enthalpy of hydration, and the lattice energy can be related as
$\Delta_{\text {sol }} \mathrm{H}^0=\Delta_{\text {lattice }} \mathrm{H}^0+\Delta_{\text {hyd }} \mathrm{H}^0$
For most of the ionic compounds, $\Delta_{\text {sol }} \mathrm{H}^0$ is positive and the dissociation process is endothermic. Therefore the solubility of most salts in water increases with the rise of temperature. If the lattice enthalpy is very high, the dissolution of the compound may not take place at all.
Recommended topic video on (Lattice Enthalpy, Hydration Enthalpy And Enthalpy Of Solution)
Some Solved Examples
Example 1: A solution of 500ml of 0.2M KOH and 500ml of 0.2M HCl is mixed and stirred. The temperature rise is T1. The experiment is repeated using 250ml of each of the solutions, the temperature raised is T2. Which of the following is true?
1) $T_1=T_2$
2) $T_1=2 T_2$
3) $T_1=4 T_2$
4) $T_2=9 T_1$
Solution
Suppose heat evolved in 1st case is Q1 and that in the second case is Q2.
$\begin{aligned} & \text { Then } Q_2=\frac{1}{2} Q_1 \quad \text { But } Q_1=1000 T_1 \\ & \text { and } Q_2=500 T_2 \\ & \Rightarrow 500 T_2=\frac{1}{2} \times 1000 T_1 \\ & \text { i.e } T_2=T_1\end{aligned}$
Example 2: Which of the following is the most favorable condition for the solubility of an ionic compound in water?
1)high lattice enthalpy and high hydration enthalpy of its ions
2) low lattice enthalpy and high hydration enthalpy of its ions
3)low lattice enthalpy and low hydration enthalpy of its ions
4)high lattice enthalpy and low hydration enthalpy of its ions
Solution
The solubility or the heat of solution of an ionic compound is related to the lattice energy and the heat of hydration of the ions

The enthalpy of solution, enthalpy of hydration, and the lattice energy can be related as
$\Delta_{\text {sol }} \mathrm{H}^0=\Delta_{\text {lattice }} \mathrm{H}^0+\Delta_{\text {hyd }} \mathrm{H}^0$
High negative values are a favorable condition for the solubility of ionic compounds
Thus, for greater solubility, the lattice energy should be low and the hydration enthalpy should be high.
Example 3: Enthalpy of solution of NaOH (solid) in water is -41.6 kJ/mol. When NaOH is dissolved in water, the temperature of water
1) increases
2)decreases
3)does not change
4)fluctuates indefinitely
Solution
When NaOH dissolves in water, then heat is released. Some part of this heat released will be used up by water and its temperature will be increased.
Example 4: Lattice energy is inversely proportional to the sum of radii of:
1)Cation
2)Anion
3)Atoms
4) Both cation and anion
Solution
Lattice energy is directly related to the product of the ion charges and inversely related to the internuclear distance (which is the sum of radii of cation and anion).
Summary
Lattice enthalpy is defined as the energy obtained from the combination of gaseous ions to make a solid ionic compound. This gives rise, in an exothermic process, to the potential strength of the ionic bonds within the lattice. One example is the case in which the formation of sodium chloride (NaCl) from sodium (Na⁺) and chloride (Cl⁻) ions is very exothermic because the ions are held together by very strong electrostatic forces. The high lattice enthalpy reflects a stable and tightly bound ionic crystal. Hydration enthalpies quantify the energy released when gaseous ions dissolve in water and they turn to hydrated ions.For example, sodium (Na⁺) and chloride (Cl⁻) ions will release energy in the way of hydration upon dissolution in water, as the ions will interact strongly with the polar water molecules.
