Leaching Process
Leaching can be defined as the process of extracting a given soluble substance by washing or dissolving it with a liquid solvent. In the activity of metallurgy, disengagement in an aqueous solution is used for the extraction of metals from mineral materials. This way, it abandons the unwanted insoluble components. This treatment works very well on the back of its being an economical method of processing low-grade ores at a large scale.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- 2. Kinds of Leaching Processes
- 3. Relevance and Applications
- Some Solved Examples
- Summary
- Solvent: A liquid containing chemicals like acids or bases prepared to dissolve the target metals.
- Ore: Naturally occurring solid material that can be used to derive a metal or valuable mineral.
- Concentrate A material that has been subjected to processing in a way that leads to an increase in the concentration of the wanted metal.
In this method, the powdered ore is treated with a suitable chemical reagent which dissolves the ore while impurities remain insoluble in that reagent.
For example:
Bauxite is separated from Fe2O3, SiO2, and TiO2, with the help of NaOH in which Al2O3 gets dissolved while the rest are insoluble.
For example: Al2O3( s)+2NaOH(aq)+3H2O(l)→2Na[Al(OH)4](aq)
The sodium aluminate present in the solution is neutralized by passing CO2 gas and hydrated Al2O3 is precipitated. At this stage, a small amount of freshly prepared sample of hydrated Al2O3 is added to the solution. This is called seeding. It induces the precipitation.2Na[Al(OH)4](aq)+CO2( g)→Al2O3⋅xH2O(s)+2NaHCO3(aq)
Sodium silicate remains in the solution and hydrated alumina is filtered, dried, and heated to give back pure Al2O3.
Al2O3⋅xH2O(s)→1470 K Al2O3( s)+xH2O(g)
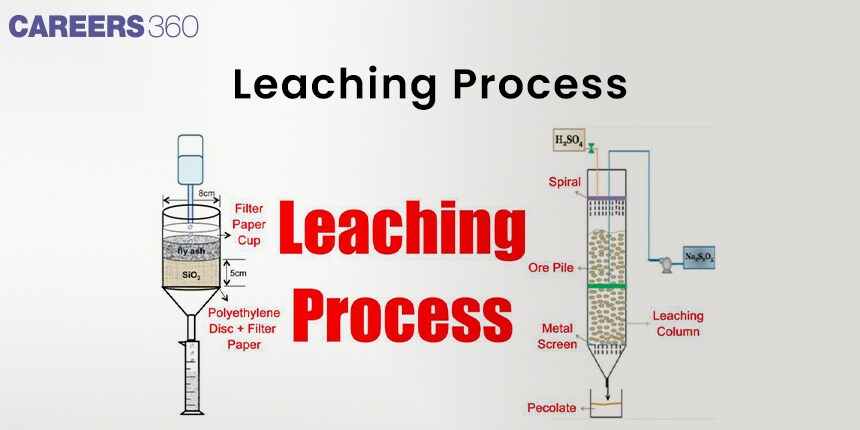
2. Kinds of Leaching Processes
Heap Leaching
Heap leaching is a common practice for the extraction of metals such as gold, copper, and uranium. The heaps are normally placed in huge heaps and a solution for leach is sprayed over the heap to dissolve metals, which get collected at the bottom.
In-situ Leaching
In-situ leaching involves directly injecting the leaching solution into an ore deposit by boring holes. The leachate then percolates through the ore deposit, hence, dissolving metals that get pumped and passed to the surface for recovery. This method is to a larger degree less disruptive to the environment compared to conventional mining.
Tank Leaching
Tank leaching is carried out in large tanks filled with crushed ore and the leaching solution. Such a procedure allows far greater control of leaching conditions than other procedures and is used for more complex ores.
Vat Leaching
Following is the process: Ore used to be placed in large vats or containers, and it came into direct contact with the leaching solution that had flooded them. This process is normally used in the case of high-grade ores, which require intensive processing.
3. Relevance and Applications
Industrial Applications
This process of leaching is used in vast amounts in the mining industry for the extraction of noble metals – such as gold and silver along with base metals like copper and iron. Leaching is comparatively a cheap and efficient technique that suits the extraction of noble metals; leaching becomes very necessary when the ore to be treated is of low grade. Leaching is also used in the extraction of heavy metals from electronic wastes by leachates in the recycling industry for resource management and pollution control.
Academic Research
The study of leaching processes today within the halls of academia is directed toward the objective of reducing the environmental impact as well as developing new methods that can effectively improve extraction. Optimally, sure the kind of research today must be all about optimization for leaching conditions, understanding of the underlying chemical reactions, and figuring out green solvents that could replace the hazardous chemicals.
Environmental Impact
Essentially, leaching processes would harbor very ill-fated hazards to the environment if good work practices are not maintained. Leaching is one more hazard of the chemicals involved throughout the whole process. Cyanide in leaching gold requires careful handling and disposal to avoid any water source pollution. In fact, studies take the lead in identifying suitable application methods of leaching agents to promote safe practices and proper handling of wastes to reduce these impacts.
Recommended topic video on(leaching)
Some Solved Examples
Example 1
Question: Which one of the following benefaction processes is used for the mineral Al2O3⋅2H2O?
- Froth floatation
- Leaching
- Liquation
- Magnetic separation
Solution: Leaching is done to concentrate ore. In this method, the powdered ore is treated with a suitable chemical reagent that dissolves the ore while impurities remain insoluble in that reagent. For example, Bauxite is separated from Fe2O3,SiO2, and TiO2 with the help of NaOH, in which Al2O3 gets dissolved while the rest are insoluble. The chemical reactions involved are:
Al2O3(s)+2NaOH(aq)+3H2O(l)→2Na[Al(OH)4]
The sodium aluminate present in the solution is neutralized by passing CO2 gas, and hydrated Al2O3 is precipitated. This process is known as seeding. The reaction is:
2Na[Al(OH)4](aq)+CO2(g)→Al2O3⋅xH2O(s)+2NaHCO3(aq)
Hence, the correct answer is option (2).
Example 2
Question: In the isolation of which one of the following metals from their ores, the use of cyanide salt is not commonly involved?
1)Zinc
2)Gold
3)Silver
4) (correct)Copper
Solution:
Silver and Gold are directly reached out using NaCN.
NaCN is used as a depressant in a mixture of ZnS and PbS.
Cyanide salt is not involved in the extraction of copper.
Hence, the answer is the option (4).
Example 3
Question: Match List - I with List - II.
List - I (A) Concentration of gold ore
(B) Leaching of alumina
(C) Froth stabilizer
(D) Blister copper
List - II (I) Aniline
(II) NaOH
(III) SO2
(IV) NaCN
Choose the correct answer from the options given below.
- (A) - (IV), (B) - (III), (C) - (II), (D) - (I)
- (A) - (IV), (B) - (II), (C) - (I), (D) - (III)
- (A) - (III), (B) - (II), (C) - (I), (D) - (IV)
- (A) - (II), (B) - (IV), (C) - (III), (D) - (I)
Solution:
- (A) NaCN is used for the concentration of gold ore. (IV)
- (B) Leaching of alumina is done by NaOH (II)
- (C) Froth stabiliser →rightarrow→ Aniline (I)
- (D) Blister copper →rightarrow→ due to the evolution of SO2 (III)
Hence, the correct answer is option (2).
Summary
Leaching is one of the basic chemical processes by which alternatives are made in the extraction of appreciated metals from various materials. The process is certainly applicable at the industrial and environmental levels, with its greatest utility found where knowledge of heap, in-situ, tank, and vat leaching routines is applied to mining, recycling, and academic work. The dissolution of metals in the process makes it a process of metal recovery, with metals getting dissolved and then refined with a solvent. This method has, however, been widely applied in the recovery of metals from low-grade ores and electronic wastes.
Also Read
06 Feb'25 11:59 PM
06 Feb'25 11:54 PM
09 Dec'24 11:00 AM
21 Oct'24 04:24 PM
07 Oct'24 02:35 PM
07 Oct'24 02:24 PM
07 Oct'24 02:17 PM
07 Oct'24 12:55 PM
04 Oct'24 07:17 PM