Specific Conductivity and Molar Conductivity - Definition, Unit, Relation, FAQs
Here in this article we will be discussing about conductance, conductivity, symbol of conductance, what is specific conductance, definition of specific conductivity, unit of specific conductivity, Specific conductivity of a solution, ratio of specific conductance to that of conductance, definition and relationship between conductivity and molar conductivity, what is equivalent conductivity and everything related to specific and molar conductivity will be discussed here.
What is meant by Conductance?
The term conductance is the reciprocal of resistance and it is denoted by the symbol G. Conductance can be defined as the measure of ease of current flow through a conductor. It can be given by the formula:
Conductance, G=1/R ……………(1)
In equation (1), ‘R’ is the resistance of the conductor. The unit of conductance is ohm-1 or Ω-1 and its SI unit is Siemens or S.
The conductance of a material generally depends on the following factors:
- The nature of the metal.
- The number of valence electrons present per atom.
- Temperature (conductance generally decreases with increase in temperature).
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Ionic conductance
The capacity of an ion to conduct electricity is commonly defined as ionic conductance. The value of ionic conductance of a metal ion is affected by the extent of its hydration in aqueous solutions.
In the state of infinite dilution, the ionization of the electrolyte will be complete and all forces of interaction between the ions will have ceased to exist. Under such a condition, all the ions that can possibly be derived from the electrolyte under consideration are free to carry current. The motion of ionic charge causes electrical conductivity. It is called ionic conductivity or ionic conductance. Equivalent conductance, molar conductance and specific conductance are different types of conductance.
Conductivity or Specific conductance
Define specific conductance
The term specific conductance, nowadays referred to as property of any conductor which is the capacity to conduct electricity.It can be represented by the symbol ‘K’. Specific conductance gives the measure of capacity of a material to conduct electricity.
Specific conductance formula or conductivity can be given as:
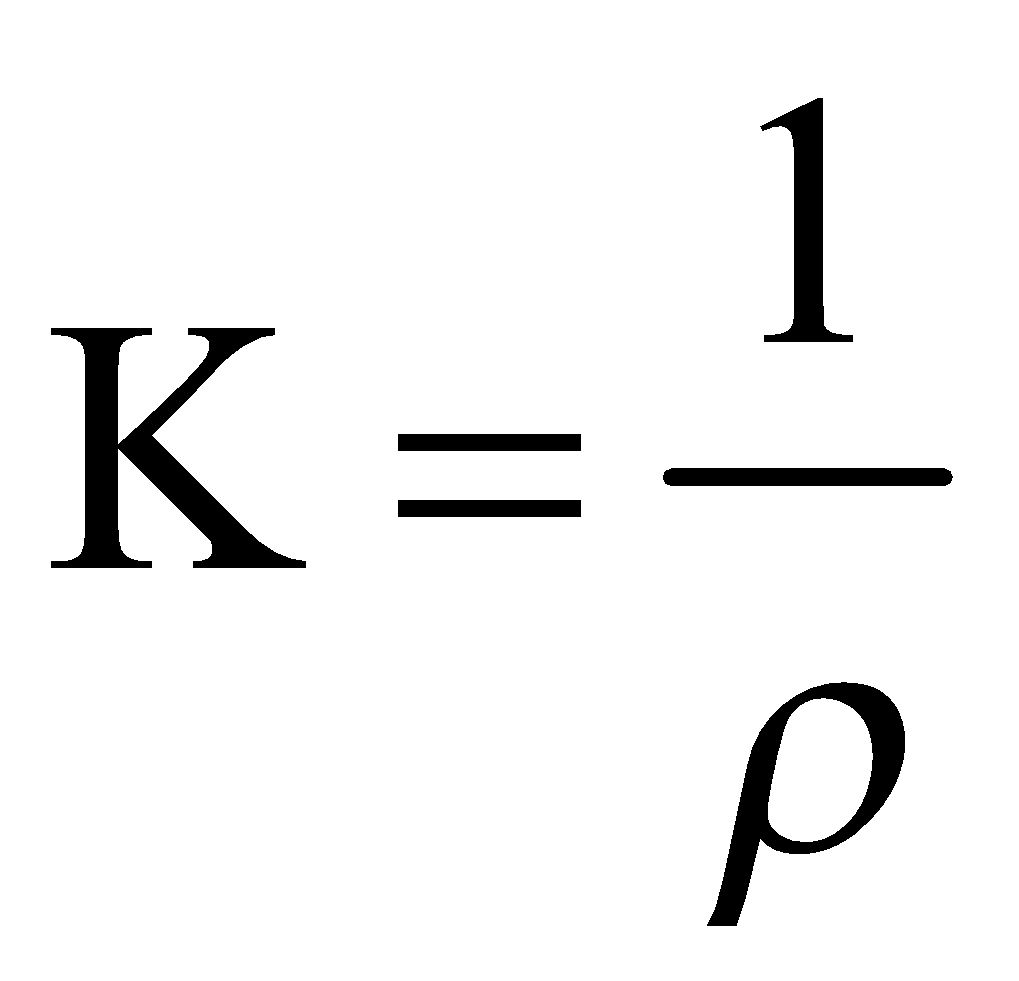
![]()
In equation (2), ρ is the specific resistance.
We know that 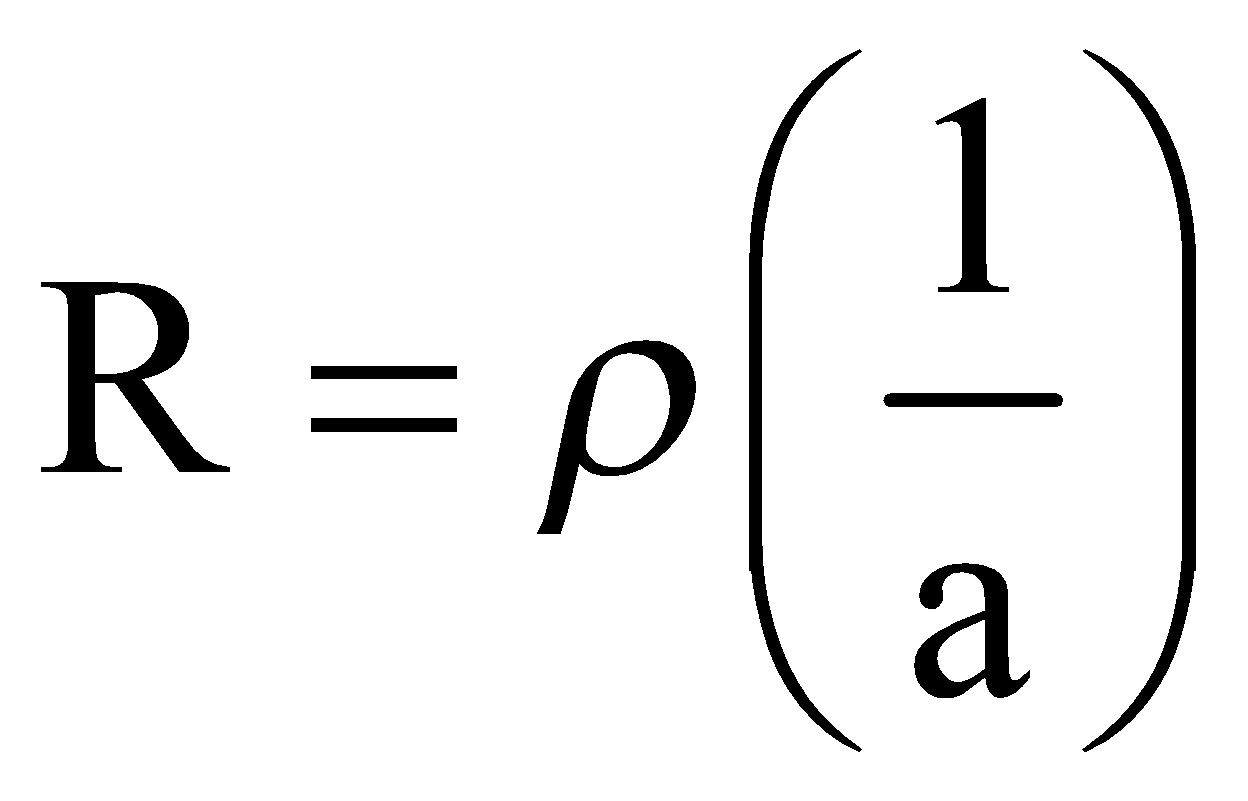
![]()
Here in equation![]() ’R’ indicates the resistance of a conductor of length ‘l’
’R’ indicates the resistance of a conductor of length ‘l’ ![]() and ‘a’ is the area of cross section in cm2.
and ‘a’ is the area of cross section in cm2.
Then,
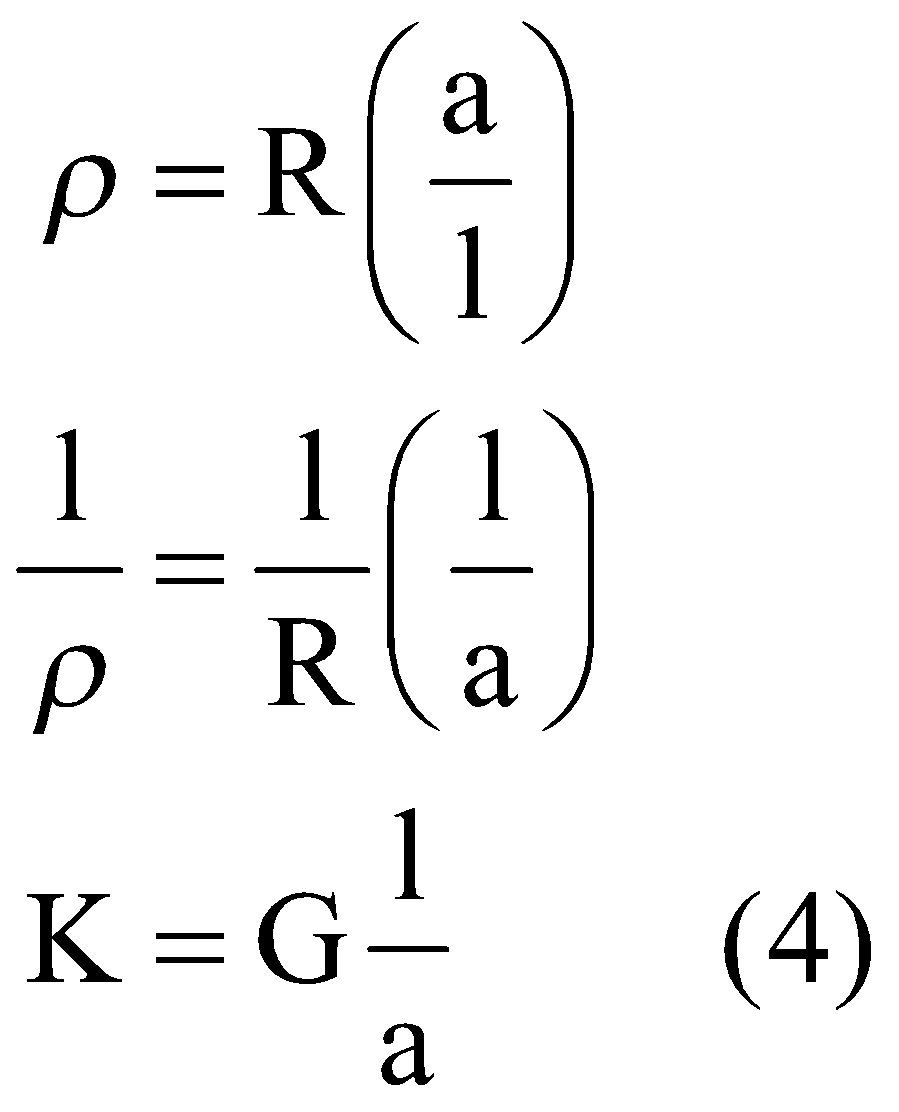
Here in equation(4) ’G’ is the conductance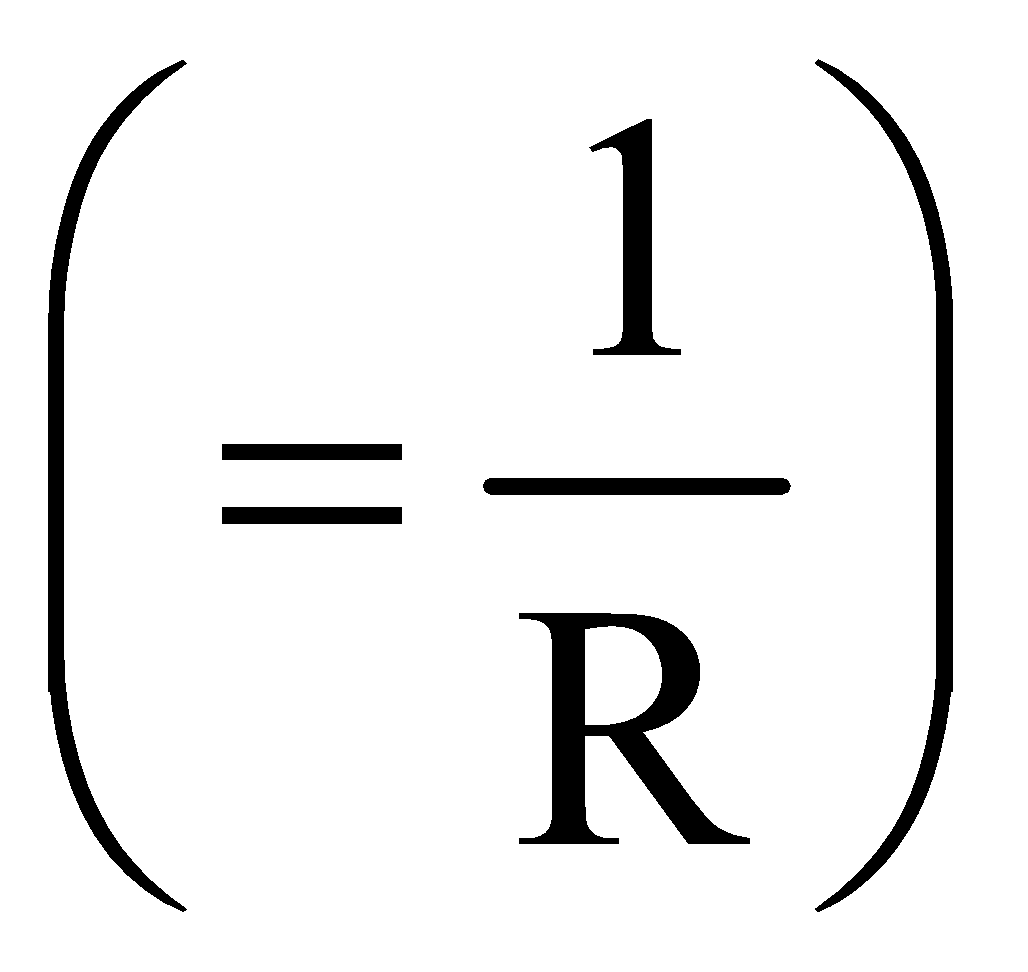 . Obviously if
. Obviously if![]() , and
, and ![]() , then equation
, then equation![]() becomes,
becomes,
![]()
Thus the conductivity or specific conductance of an electrolyte solution represents the conductance with unit length and unit cross section. In other words, conductivity or specific conductance of an electrolyte solution represents the conductance of one centimeter cube of the solution kept between two ‘![]() ’ electrodes of unit area of cross section and placed unit distance apart.
’ electrodes of unit area of cross section and placed unit distance apart.
The unit of specific conductance is microsiemens/cm
- The conductivity or specific conductance of an electrolyte depends on the following factors.
- Nature of electrolyte – Strong electrolytes have high conductance whereas the weak electrolytes have low conductance.
- Concentration of the solution – Molar conductance varies with concentration of the electrolyte.
- Temperature – The conductivity of an electrolyte increases with increase in temperature.
What does the term cell constant indicate?
The term cell constant is obtained by dividing the distance between the two electrodes in a conductivity cell by the cross-section of the electrode. It is commonly expressed in the unit ![]() and its SI unit is
and its SI unit is![]() .
.
The expression for conductivity of an electrolyte solution is given as:
![]()
i.e., Conductivity ![]() Conductance
Conductance ![]() Cell constant
Cell constant
The cell constant ![]() can also be denoted as
can also be denoted as![]() . Then the expression for ‘K’ becomes:
. Then the expression for ‘K’ becomes:
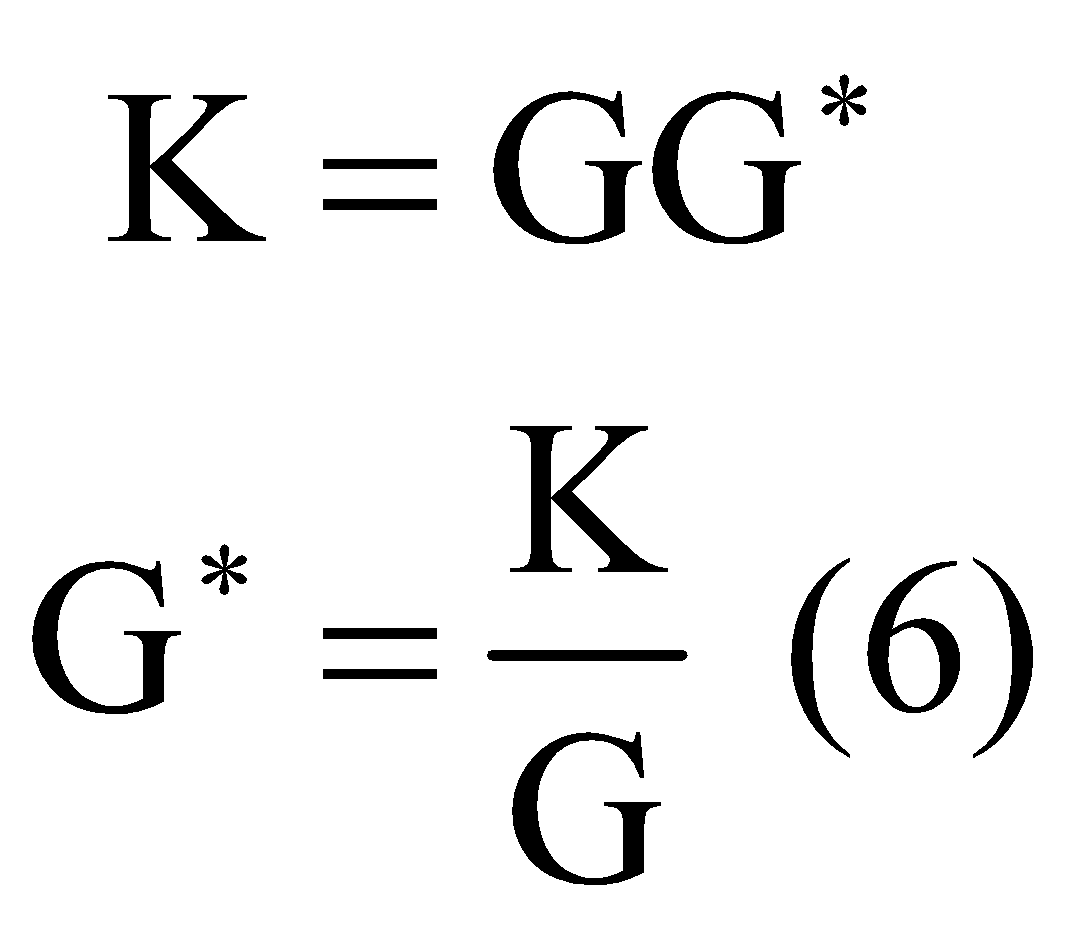
Hence from equation ![]() It is clear that the cell constant is the ratio of specific conductance and conductance.
It is clear that the cell constant is the ratio of specific conductance and conductance.
Related Topics Link, |
Equivalent conductivity or Equivalent conductance
Equivalent conductivity of an electrolyte solution of a given concentration is explained as the conducting power of ions formed from one equivalent of electrolyte present in solution. It is generally denoted as![]() .
.
Specific conductance or conductivity (K) of a solution is related to equivalent conductivity by the equation:
![]()
Here in equation ![]() ’K’ is expressed in
’K’ is expressed in ![]() and the concentration ‘c’ in
and the concentration ‘c’ in![]() . Then the unit of
. Then the unit of ![]() is
is ![]() .
.
If ‘N’ is the normality of the solution and ‘K’ is expressed in ![]() then,
then,
![]()
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 3 Electrochemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry
- NCERT notes Class 12 Chemistry Chapter 3 Electrochemistry
Molar conductivity or Molar conductance
Molar conductivity or molar conductance of an electrolyte solution of a given concentration can be defined as the ratio of conductivity and the molar concentration. It is denoted as![]() .
.
Specific conductance or conductivity (K) of a solution is related to molar conductivity by the equation:
![]()
In equation![]() , when ‘K’ is expressed in
, when ‘K’ is expressed in ![]() and the concentration ‘c’ in
and the concentration ‘c’ in![]() , then the SI unit of
, then the SI unit of ![]() will be
will be![]() .
.
If ‘M’ is the molarity of the solution and ‘K’ is expressed in ![]() then,
then,
![]()
![]()
- For an electrolyte solution,
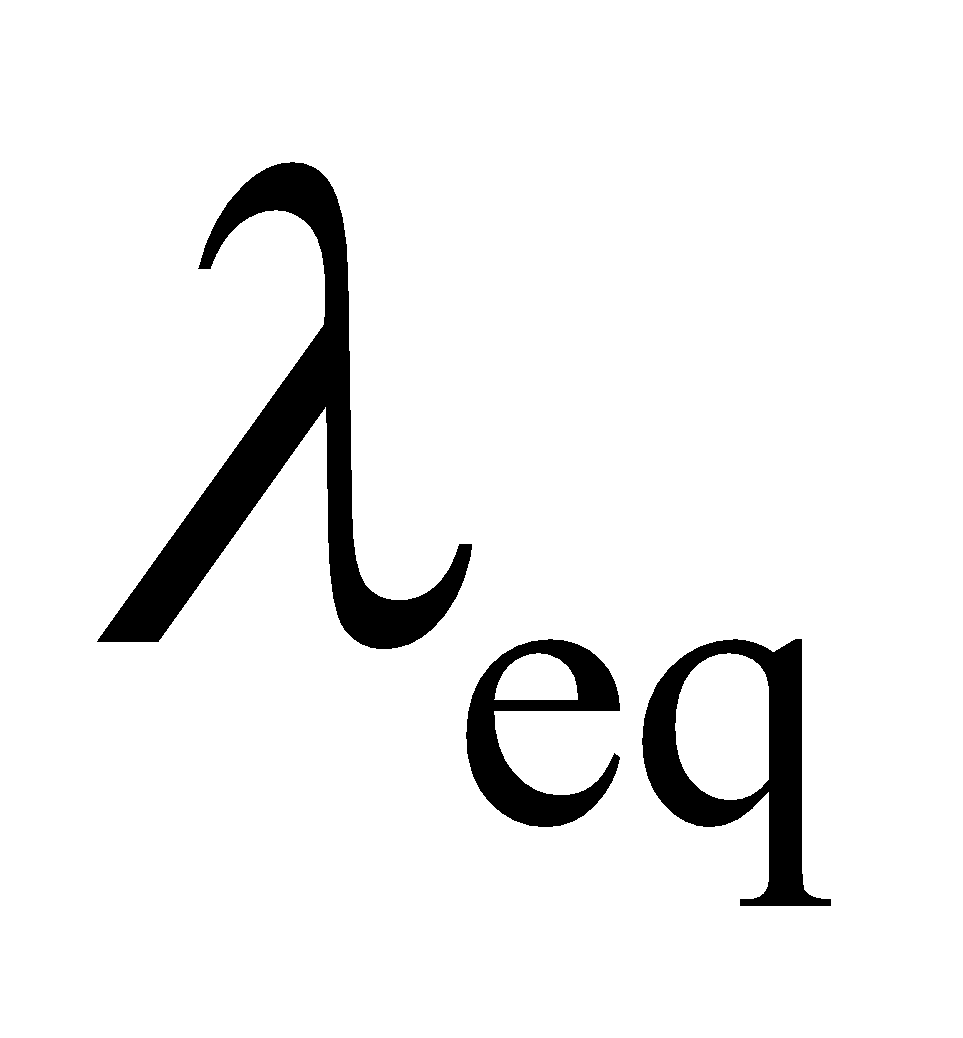 is related to
is related to 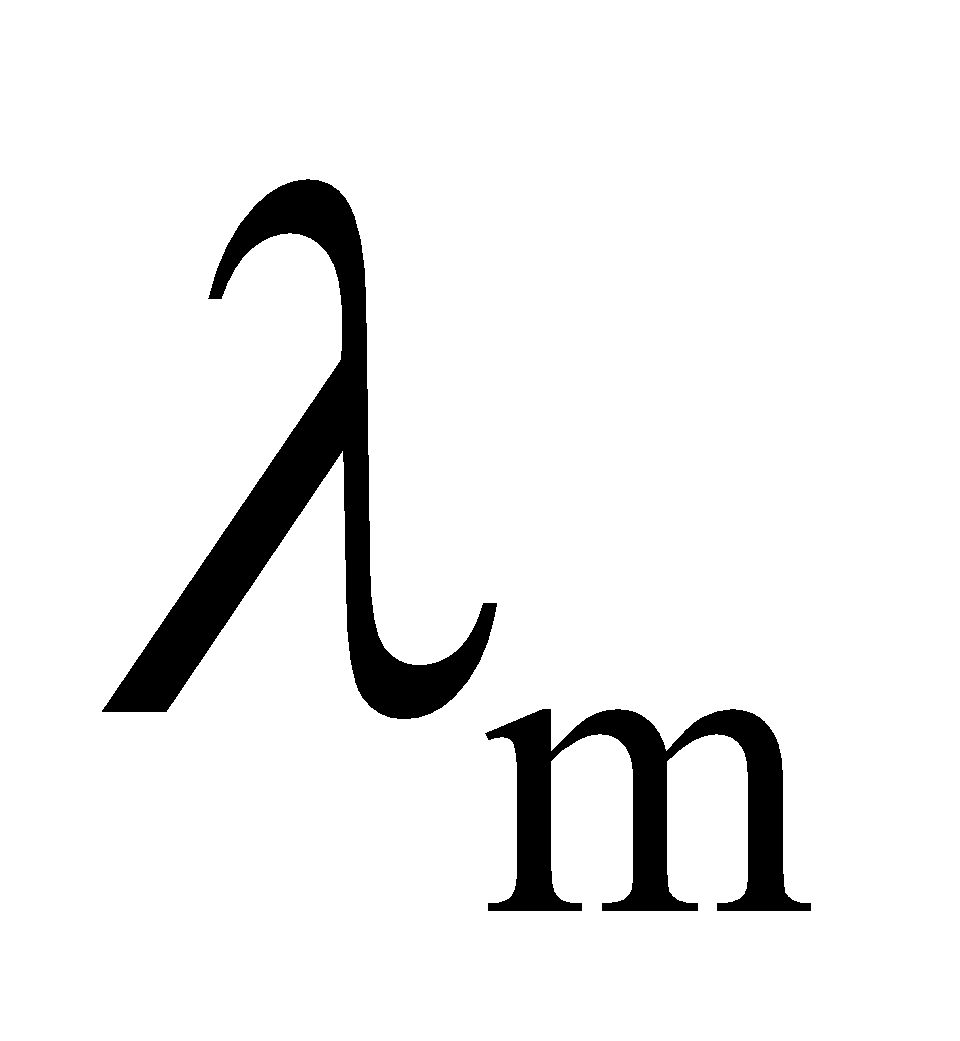 as:
as:
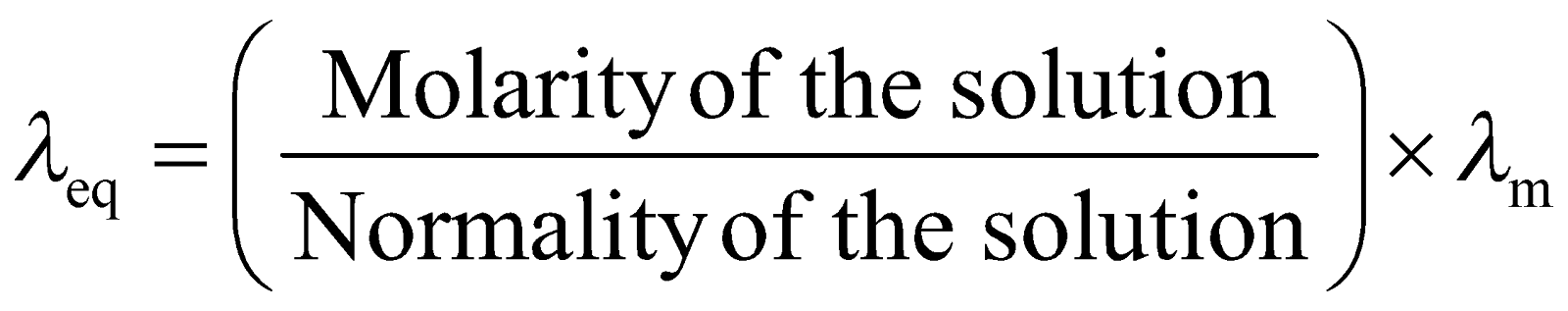
![]()
Relation between conductivity and molar conductivity
Λm=KC
C is the concentration
K is the conductivity
Λm is the molar conductivity
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: