Sublimation - Definition, Examples, Process, FAQs
Define Sublimation with Example.
Sublimation definition chemistry and Sublimation meaning: Sublimation refers to the passage, change, or conversion of substances from one state to another, for as from a solid phase to a gas phase.

Define sublimation: Sublimation is defined as the transition of a substance from a solid to a gaseous state without converting to a liquid state. This is an endothermic phase transition that takes place at a temperature and pressure below the substance's triple point.
At different temperatures, elements and compounds have three distinct states.
Solid-state to liquid-state and liquid-state to gaseous-state transitions are required for the transition from solid to gas examples state.
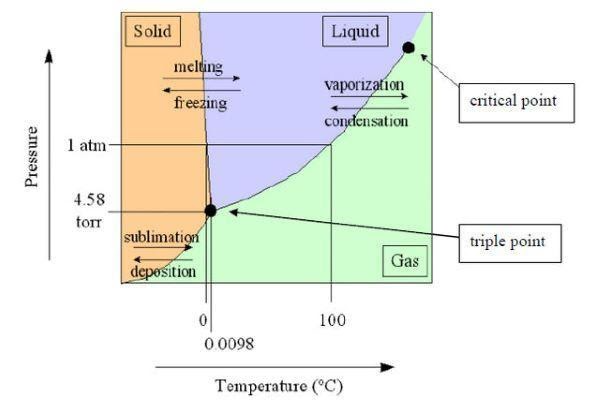
Solids can immediately sublime into the air if their vapour pressure is high enough at a certain temperature.
Sublimation occurs in solids with a high pressure at their triple point.
Triple Point : The triple point is the temperature and pressure of a substance that allows it to exist in all three states of matter at the same time. The triple point is a distinguishing feature of a substance.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs
- Define Sublimation with Example.
- The Process of Sublimation
- Conditions for Sublimation to Occur
- Sublimation Diagram
- What is a Sublime?
- Definition of Sublimate?
- Sublimation Examples
- What is Desublimation or Reverse Sublimation?
- Some Examples of Reverse Sublimation or Deposition
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
The Process of Sublimation
When some of the molecules absorb heat energy, they gain significantly more energy than their neighbours, overcoming the laws of attraction and escaping into the vapour phase. This is referred to as an endothermic reaction since it demands additional energy.
Enthalpy or Heat of Sublimation- The enthalpy of sublimation is the amount of heat or energy necessary to alter a substance from solid to gaseous. It is usually stated in KJ/mol or even KJ/kg.
Sublimation is only possible with a small number of solids. As a result, the sublimation process can be employed as an excellent purifying procedure. This is a very good approach for separation and purification when a solid is contaminated with non-volatile contaminants. In a vessel, the contaminated or impure substance is heated while remaining in touch with a cool surface.
The volatile solid sublimes and adheres to the cool surface above it as it heats up, while the impurities remain below. This is a very environmentally friendly procedure because it does not require any solvents and produces no trash. Its main drawback is that it is ineffective at separating volatile substances from one another.
Conditions for Sublimation to Occur
First, the sample must be held at a high enough temperature to sustain a high vapour pressure; otherwise, the substance may break down if it falls below that temperature.
Second, the sublimed vapour must be able to condense or solidify on a surface.
Sublimation Diagram
Related Topics link, |
What is a Sublime?
Sublime is the name given to a solid substance that transforms into a gas.
Definition of Sublimate?
Sublimate is the solid that results from cooling vapour.
Sublimation Examples
Dry ice, a frozen form of carbon dioxide, is the greatest illustration of sublimation. When dry ice is exposed to air, it immediately transforms from a solid to a gaseous state, which appears as fog. The gaseous state of frozen carbon dioxide is more stable than the solid state.
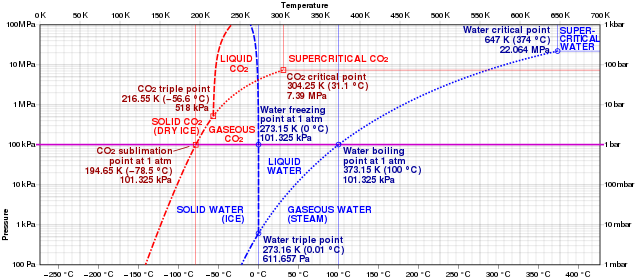
The above given figure shows the comparison of carbon dioxide and water phase diagrams. The transformation from dry ice or solid carbon dioxide to gaseous carbon dioxide can be perfectly seen.
Sublimation occurs when carbon dioxide transforms from a solid to a gas with no intervening liquid form at pressures below 5.13 atm and temperatures below 56.4 °C (216.8 K; 69.5 °F) (the triple point).
Also read :
- NCERT notes Class 11 Chemistry Chapter 5 States of Matter
- NCERT solutions for Class 11 Chemistry Chapter 5 States of Matter
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter
The enthalpy of sublimation of dry ice is 571 kJ/kg (25.2 kJ/mol)
Naphthalene, an organic chemical, is another well-known example of sublimation. Pesticides containing naphthalene, such as mothballs, are common. The presence of non-polar molecules linked together by Van Der Waals intermolecular interactions causes this organic complex to sublime. At 176 degrees Fahrenheit, naphthalene sublimes and forms vapours. It forms needle-like crystals when exposed to chilly surfaces.
In the field of forensic science, sublimation is used. Dye-sublimation printers aid in the production of digital images in a detailed and realistic manner, which aids in substance analysis. Sublimation is a popular process for purifying volatile chemicals among chemists.
Dye sublimation is a relatively eco-friendly and worker-safe method. There is no waste generated during this operation. When compared to screen printing on garments and apparels, this procedure produces no wastewater. During the heating process of the inks, there is a risk of fumes being released, which can be hazardous.
What is Desublimation or Reverse Sublimation?
Desublimation, also known as deposition, is the process by which a gas is turned directly into a solid state.
NCERT Chemistry Notes:
Some Examples of Reverse Sublimation or Deposition
Beaches, deltas, glacial moraines, sand dunes, and salt domes are some of the examples of reverse sublimation or deposition. When water vapour in the air comes into contact with a window in extremely cold conditions, it rapidly freezes without ever producing liquid water, resulting in frost on the window.
Also check-
Frequently Asked Questions (FAQs)
The transition in sublimation is from the solid to the gas phase, whereas in evaporation it is from the liquid to the gas phase.
Sublimation is a type of phase transition, or change in state of matter, that includes melting, freezing, and evaporation. Sublimation is the process of converting a solid to a gas without going through the liquid phase. Dry ice, or heavy carbon dioxide, is a common example of sublimation.
The air fresheners used in the bathroom. The strong subdues and releases the pleasant odour in the bathroom over a period of time. Mothballs made of naphthalene are used to keep moths and other pests away from your home.
At typical temperatures and pressures, a variety of materials, including water, iodine, arsenic, and solid carbon dioxide (dry ice), can sublimate. Other materials can be made to sublimate by putting them in a low-pressure environment.
Sublimation separates a mixture of solids, one of which is sublime. On heating, many liquids transition from solid to vapour without passing through the liquid state. Sublimation is the term for this procedure.
The amount of energy that must be delivered to a solid mole at constant pressure to turn it directly into a gas (without going through the liquid phase) is known as the sublimation molar heat (or enthalpy).
Also Read
19 Feb'25 11:49 AM
19 Feb'25 11:23 AM
19 Feb'25 11:14 AM
19 Feb'25 10:31 AM
18 Feb'25 12:15 PM
18 Feb'25 11:59 AM
09 Feb'25 10:16 PM
08 Feb'25 05:31 PM
08 Feb'25 05:28 PM
07 Feb'25 11:56 PM