Zone Refining - Definition, Principle, Process, Applications, FAQs
Zone Refining - Everything today we use is generally made up of metals. Hence the increasing demand for metals also increases the need for the purity of metals. For producing semiconductors we need the purest form of silicon and germanium. So there is a need of purifying these metals. Metallurgy is defined as the science of extracting metals in their pure form from their ore. Scientists discovered various methods for the purification of metals, zone refining is one of them. In 1951 scientist William G. Pfann give us a method to purify metals that is known by the name zone refining.
This Story also Contains
- What Is Zone Refining?
- Principle Of Zone Refining
- Zone Refining Process
- Applications of Zone Refining
- Limitations of Zone Refining

It is one of the most important steps which is used for the development of transistors and for the whole electronics era. Before the discovery of zone refining purification of metals, was done with a process called progressive freezing. Zone refining is also known by some other names like zone melting, traveling melting zone, and floating zone process. The zone refining process was first discovered by the scientist we can say first used by a scientist name Desmond Bernal and this process is first used on the metal germanium. Silicon is also one of the known metals but in this process, electrical heating coils will not work properly due to their high melting point.
Also read -
What Is Zone Refining?
Zone refining is said to be a technique and zone refining is used for getting highly pure crystals of impure metals or elements but especially this process is used for metals with the help of melting and crystallization processes. it is a method of separation that is used to separate pure metals. In this process, the first crystals were heated by which all the impurities present between crystals melt and it forms a molten zone that moves along with crystals and provides us a pure form of metals. Hence we can say that zone refining is used for the purification of metals.
Principle Of Zone Refining
Zone refining is based on the principle that impurities of metal are more or we can say easily soluble in their molten state due to this property when a molten metal turns into crystals during the crystallization process impurities are removed automatically as these are not able to form pure crystals. Impurities can be easily removed in the molten zone easily with the help of zone refining through the movement of the moving heater and in this process, the recrystallized pure form of metal is left behind in the solid state. During this zone should be moving as slowly as possible which will develop highly pure metal.
It may also be noted that the segregation coefficient in the case of a zone refining process should be less than one whereas the segregation coefficient can be defined as the ratio of impurity present in a solid state to impurity present in a liquid or melting state. This examines that when conditions are exactly set towards the solid-liquid boundary then the impurities present between the atoms tends to diffuse into the liquid region.
Zone Refining Process
The zone refining process can be easily understood with the help of the zone refining diagram given as follows:
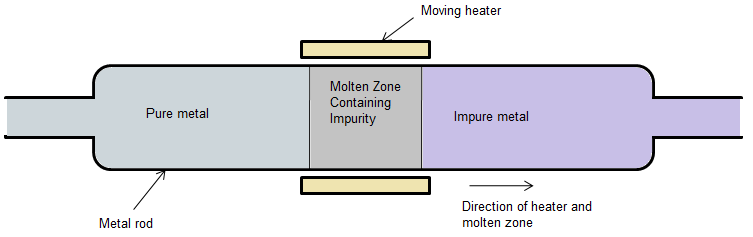
In the zone refining process moving heater or we can say it mobile heater is present at one end and on the other end, impure metal is present. This impure metal rod is fitted with a mobile heater which further exists in a circular manner in such a way that this column contains an inert gas in it. With the help of this circular heater, heat is produced which heats the rods and develops a region in which temperature is increasing up to its melting point in such a way that the plane in which it is present is perpendicular to the axis of the rod. With the movement of the heater along its rods the molten zone also propagates down the rods.
Make sure the movement of the heater is very slow because of these impurities and pure metal both get melted but atoms of pure metal will recrystallize on the other hand molten impurities or we can say the molten zone move with respect to this heater. After this whole process impurities which are in the solid form will remain at one end and pure metal remains on the other end. To get the purest form of metal we have to repeat the process several times. This process is very useful for removing impurities of semiconducting elements like germanium, gallium, silicon, etc. The zone refining process is also for refining high-purity metals.
Related Topics,
- Chemical Properties of Metals
- Method of Separation
- Fractional Distillation
- Ores and Minerals
- Types of Minerals
Applications of Zone Refining
There are many applications of zone refining which can be explained as follows:
1. The main application of zone refining which is widely used is the purification of metals.
2. It is also a very effective process that is used for the removal of impurities from semiconductors. The main examples of semiconductors are Germanium (Ge), Gallium (Ga), and Silicon (Si).
3. There is a process called the float zone refining process which is used in solar cells.
4. It is used for the preparation of organic as well as inorganic compounds.
5. It is mentioned by many scientists that the zone refining process is highly useful in preparing organic chemical standards used for HPLC i.e. High-performance liquid chromatography, absorbance, or fluorescence spectroscopy.
6. Zone refining is also useful in analytical techniques in which we need substances of the highest purity which are further used for standardization and calibration of instruments.
7. Zone refining is used in the crystallization process which is used to concentrate an enzyme, peptide, antibiotic or those substances which are thermally unstable in nature.
8. This method is also used in aqueous solutions for the concentration of heat-labile biological materials.
Thus we can say that there is a wide range of applications present for zone refining, therefore this process is in much demand nowadays.
Rather than applications, some limitations can also be seen in the zone refining process which can be discussed below:
Limitations of Zone Refining
1. Zone refining is not economically balanced i.e. this process is really expensive in nature due to this its applications are limited to laboratory reagents and for valuable chemicals only.
2. Sometimes we face a situation where for all impurities solid-liquid equilibrium is not favorable then in this situation we have to combine zone refining with some other techniques to attain ultrahigh purity in metals. Which makes it more difficult and costly in nature.
Also, students can refer to,
- NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of
elements - NCERT Exemplar Class 12 Chemistry Solutions Chapter 6 General Principles and Processes of isolation of
elements - NCERT notes Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of elements
Zone Remelting
Other than zone refining one similar term zone remelting is also used. This technique is used when two solutes are distributed through a pure metal. This process is generally useful in the preparation of semiconductors in which these two solutes carry opposite conductivity to each other. The main example of this process is in germanium, pentavalent elements of group V like antimony and arsenic produce negative conduction i.e. n-type, and trivalent atoms of group III like aluminum and boron produce positive conduction said as p-type conduction. During this process, the melting portion is distributed in such a way that it can easily form desired n-p or p-n junctions.
Also, check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
